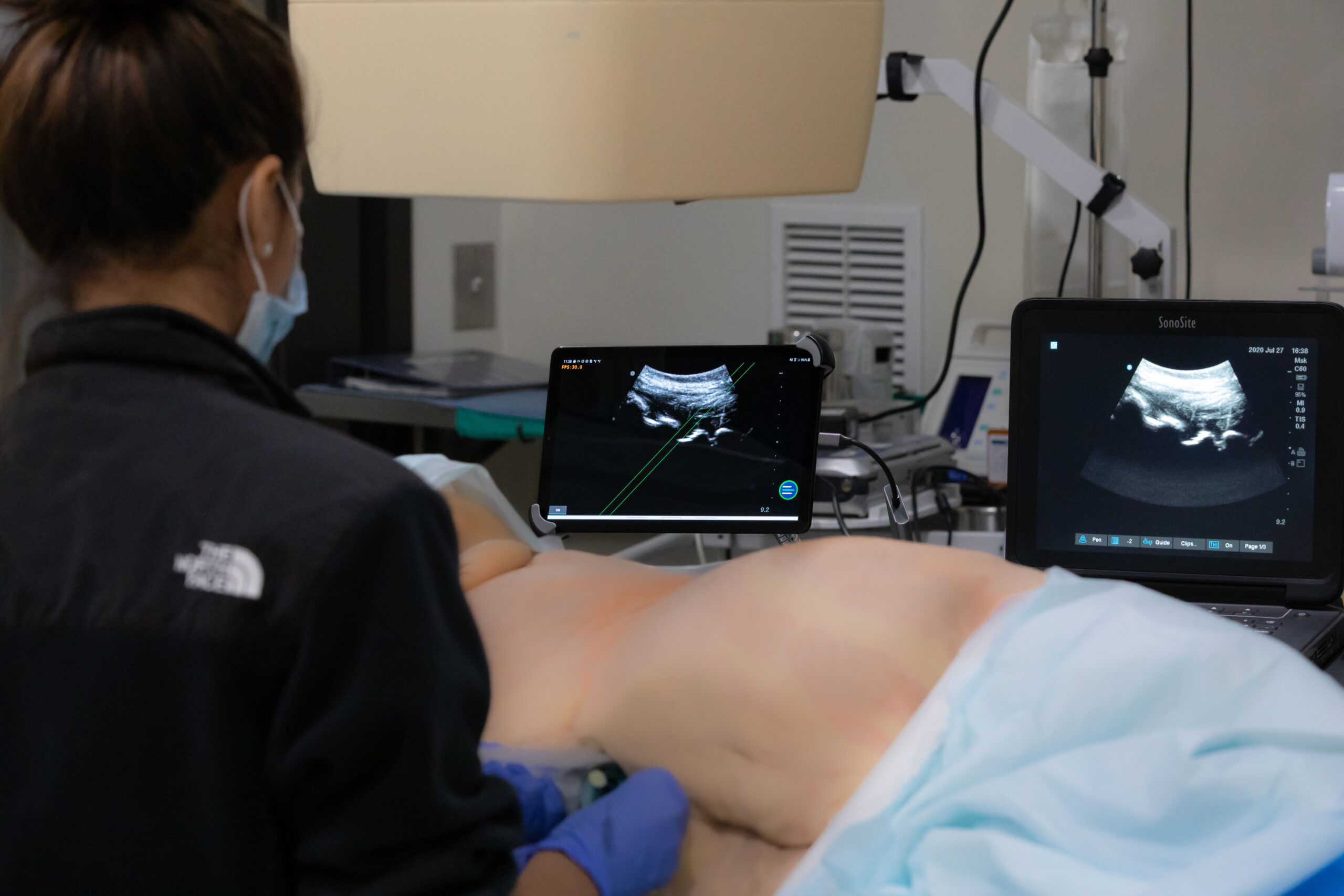
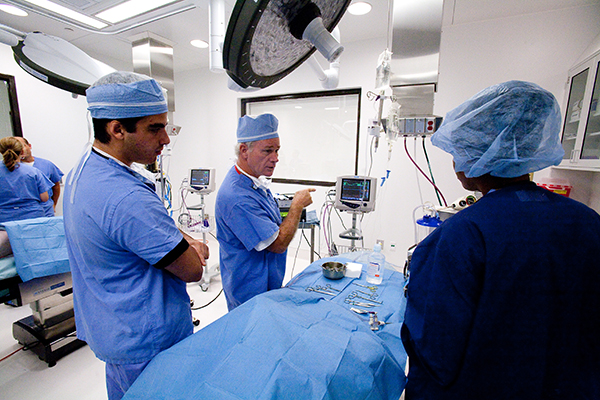
Our orthopedic preclinical testing experience includes spine, large joints, and extremities treated with devices, biologics and cell-based products. Models include, but aren’t limited to: Spine fusion, degenerative disc disease, orthopedic infection, tendon repair/replacement, fracture healing and critical-sized defect models. Our GLP and AAALAC accredited preclinical CRO facility includes high quality operating rooms and all of the equipment needed, including Digital x-ray, Micro-CT, MRI and CT, surgical instrumentation and qualified plastic histology labs. Model development is available.