Since the first quarter of 2020 the demand for virtual bioskills training and education programs for medical technologies has skyrocketed . Teams leaned on virtual engagement technologies, dedicated lab staff, and proctors onsite, to deliver the training and education required to learn how to safely employ new technologies, products and techniques. At the importance of continued education and the undeniable hands-on experience, doctors, nurses, allied healthcare professionals, and students are starting to return to in-person programs in greater numbers for bioskills training and education at T3 Labs.
What, then, do medical technology and device innovators need to know about the current state of bioskills training and education? T3 Labs’ Training and Education Program Manager Rhonda Lynch shares the following.
“More so now than pre-pandemic, planning and flexibility are essential in this time of restrictions and supply chain management challenges,” Rhonda said. “My goal is to provide a program that allows the expectations to be met for not only my direct customer, but also my customers’ customer.”
Achieving this causes Rhonda to rely on collaboration from multiple partnerships inside and outside the organization.
“My customers think of me as an extension of their own organization. This is especially true when so many of GCMI’s clients are from out of state and even out of the country. In exchange for their planning and flexibility, I receive the trust of my sponsors that I will ensure they have everything they need to accomplish their goal.”
Bioskills labs did not stop operating through the pandemic and while virtual programs increased to meet the demand of the 2020 situation, the provision of hands-on and face-to-face experiences remain a core component of our training and education programs.
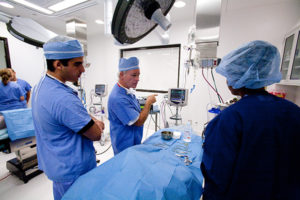 “When it comes to our lab environment, our operating rooms provide a simulated clinical environment with which our sponsors and their physicians are intimately familiar.”
“When it comes to our lab environment, our operating rooms provide a simulated clinical environment with which our sponsors and their physicians are intimately familiar.”
This became crucial when due to COVID hospital visitor restrictions tightened. The traditional challenge to have sales introduce a new device or end users to evaluate a new technology, became impossible. Bioskills provided the hospital environment without the complication and added risk.
New technologies and procedures were able to continue development and training in spite of the hurdle. The bioskills training & education program saw an enormous amount of new PPE and COVID related technologies being evaluated.
“This was a really exciting time, because our front line workers and the general public began to get creative and come up with ideas to fight COVID,” Rhonda said. “With travel and other safety restrictions easing for the moment, we are seeing steady increases in the number of participants for each program we execute for our sponsors and clients. While some of our sponsors ceased training entirely during the height of the pandemic, those that did not, saw an average of eight to ten participants in on-site programs, including the necessary T3 Labs staff. Those numbers are steadily increasing and currently stand in the 20-25 participants per program range.”
Rhonda says that surgery is a team approach and it is just as important for all involved to have training on their individual roles. Because of this, T3 Labs’ Bioskills program sees training not only focus on the surgeons, but all end users such as nurses, allied healthcare workers, first responders, students, as well as others that need hands-on experience. For instance, procedural or equipment specific training allows the nurses and techs to proficiently assist a surgeon in the OR, which potentially leads to less errors and better patient outcomes.
“In terms of technology, we’re seeing an increase in surgical robotics that enable physicians to perform procedures remotely,” she said. “We’re also seeing new technology that limits radiation exposure, including hand held devices for imaging. These new devices help limit the amount of radiation exposure that surgeons and patients are currently receiving by using fluoroscopy.”
Bioskills training and education is not immune to supply chain challenges
Rhonda said that shortages of cadaveric tissue is having a direct impact on lead times and program planning requirements, and has also instigated changes in the approach to surgical models, including increased use of animate and simulator models.
“Before the pandemic, tissue banks could fill orders with only a weeks’ notice, allowing training programs to be executed within a week or two,” she said. “Currently, tissue banks are finding it more difficult to provide the needed specimen in the usual requested time, and are now asking for at least a 30 days notice. Due to safety and testing requirements there has been an overall decrease in eligible donors due to COVID. Along with the shortage of cadaveric tissue, there are a lot of moving parts and people that go into planning a program.”
Sourcing of specialty equipment, instruments, supplies and expertise all takes time, so Rhonda’s suggestion is to plan your training and education programs as far ahead as possible and be flexible with days, as well as times.
“We can also accommodate difficult schedules by offering evenings and weekend programs, something we have always done. This has become even more valuable to our customers given the immense strain on physicians’ in-clinic responsibilities and the challenges to get into the hospitals.”
Beyond the bioskills training ‘basics’
“GCMI is a ‘full circle’ medical technology innovation supporter,” Rhonda said. “At our T3 Labs location, we not only offer bioskills training and education, design and development but also preclinical testing. We are a one stop shop for medical technology innovators, from the earliest stages of ideas and designs, through preclinical testing, and into training to prepare for clinical trials or product launch. Our full circle service and partnerships sets us apart from other labs. For those early in their innovation journey exploring functionality or those perfecting the techniques and design in R&D, we can provide the support needed to determine viability to move forward. I then shepherd those innovators forward into our Phase 0 process designed to ensure capital efficiency in medtech innovation. Bioskills are crucial throughout the entire life cycle of the product and process. Providing access to the models, services, data and the environment needed to build confidence that the idea can become a viable medical device.
 “There is a huge opportunity in Bioskills to provide exposure. Through our education program at T3 we provide programs catered to students as young as middle school age, focusing on STEM education. We take great pride in helping these young students become more aware of different healthcare avenues that they may never know of otherwise. We also support Georgia Tech’s School of Biomedical Engineering Capstone programs, allowing these students access to our facility and staff, to help evaluate, design and test their technologies that may well save untold numbers of lives in the future.”
“There is a huge opportunity in Bioskills to provide exposure. Through our education program at T3 we provide programs catered to students as young as middle school age, focusing on STEM education. We take great pride in helping these young students become more aware of different healthcare avenues that they may never know of otherwise. We also support Georgia Tech’s School of Biomedical Engineering Capstone programs, allowing these students access to our facility and staff, to help evaluate, design and test their technologies that may well save untold numbers of lives in the future.”
About GCMI and T3 Labs
GCMI is a medical device product development company. Our preclinical testing and bioskills arm, T3 Labs, provides preclinical testing services and bioskills training & education. GCMI works across a wide array of clinical therapeutic areas. We support projects from early concept feasibility through regulatory approval and commercialization.
Why GCMI? It’s all about flexibility, accountability, quality, compliance and accreditation, our scientific acumen and commitment to value. Learn more.
