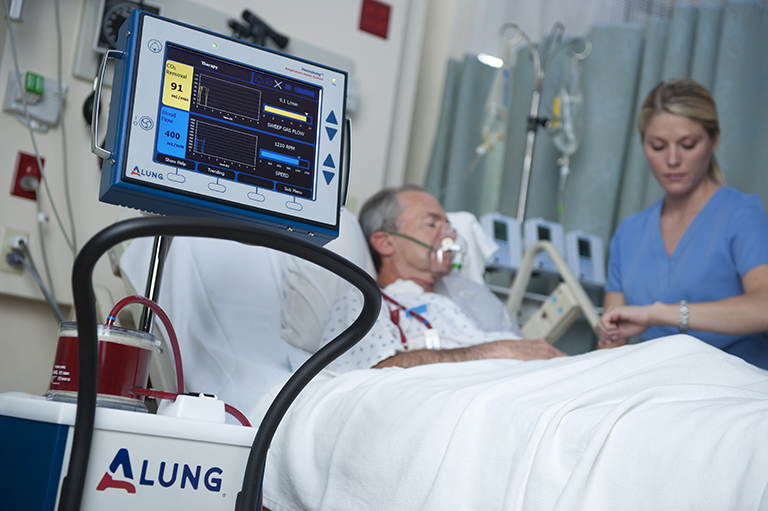
A long-time customer of GCMI and T3 Labs, ALung Technologies, has recently announced that they have received FDA Emergency Use Authorization (EUA) for their Hemolung® Respiratory Assist System (RAS), the world’s first fully-integrated respiratory dialysis system. This system removes carbon dioxide directly from the blood, allowing the patient’s lungs to rest and heal while avoiding the harmful effects of mechanical ventilation.
“Given that COVID-19 appears to share some pathophysiologic behavior with acute respiratory distress syndrome (ARDS), and the Hemolung has been used outside of the United States to treat ARDS patients, there was reasonable confidence that ECCO2R using the Hemolung could potentially benefit severely ill COVID-19 patients,” said Jeremy Kimmel, PhD, Vice President of New Technologies for ALung.
ALung published a release on the FDA’s decision:
“[T]he FDA stated that it believes the Hemolung RAS has the potential to treat lung failure as an adjunct to noninvasive or invasive mechanical ventilation, to reduce hypercapnia and hypercapnic acidosis due to COVID-19, and/or to maintain normalized levels of partial pressure of carbon dioxide(PCO2) and pH in patients suffering from acute, reversible respiratory failure due to COVID-19 for whom ventilation of CO2 cannot be adequately, safely, or tolerably achieved and, in turn, may provide clinical benefit, and that there is no adequate, approved and available alternative to the emergency use of the Hemolung RAS to treat lung failure caused by COVID-19.
‘We are pleased with the FDA’s recognition that the Hemolung may be beneficial in the treatment of COVID-19 by removal of CO2 directly from the blood during extracorporeal therapy,’ said Mr. Peter M. DeComo, Chairman and CEO of ALung Technologies.”
This EUA can be considered particularly notable because, although there have been quite a few similar authorizations granted amidst the COVID-19 pandemic, the majority of them have pertained to testing for the virus rather than treatment.

Jeremy Kimmel
“Since ALung already has an active IDE for the Hemolung, FDA was very familiar with the device’s preclinical data including our GLP study, bench data, etc,” Jeremy Kimmel, PhD, Vice President of New Technology for ALung said. “The primary rationale for FDA approving the EUA was clinical evidence that the device could potentially benefit COVID-19 patients, and that such benefits likely outweighed the risks of Hemolung therapy.”
The relationship between GCMI and ALung began back in 2013 when the company chose to partner with T3 Labs for preclinical studies supporting U.S. regulatory approval for the Hemolung RAS. ALung later selected T3 Labs as the GLP preclinical CRO partner in support of their IDE application for the Hemolung RAS in March 2017. Kimmel shared with us that having IDE approval certainly provided confidence to the FDA regarding the performance and safety of the Hemolung RAS, along with the clinical data that has been collected on the device outside of the U.S.
When it comes to what other medtech innovators can learn from ALung’s experience with GCMI and T3 Labs, Kimmel stated, “A good preclinical partner must be able to adapt to the needs, schedule and budget for a specific project. T3 Labs, now part of GCMI, has been an outstanding partner in supporting ALung throughout many preclinical studies.
“Preclinical work is always filled with unexpected outcomes – some good, some bad. Having a trusted preclinical partner that has the experience, expertise, and flexibility to work with your specific product, team, timeline and budget is of the utmost importance.”
Stories like ALung’s reinforce the importance of GLP studies when it comes to medical product development and regulatory submissions. As they can often make or break your medical product and are extremely costly, it’s crucial for innovators to take the time to fully prepare for the GLP process.
“Don’t rush into preclinical studies,” Kimmel advises aspiring medtech innovators. “Spend the necessary time to work out the kinks in your device on the bench before sinking considerable cost and resources into preclinical studies. Make sure you have a well-vetted plan so that you don’t end up biting off more than you can chew.”
ALung continues to partner with GCMI and T3 Labs for preclinical studies related to new product development, and they have plans to submit results from their pivotal, randomized controlled trial to the FDA for market approval.
“Although outcomes for use of Hemolung therapy for critically ill COVID-19 patients are still preliminary, we are hopeful that positive data from our EUA experience will support ALung’s forthcoming FDA submission,” Kimmel added.
GCMI is pleased to have a hand in bringing medtech innovation to market during this time of crisis.
You can learn more about our efforts by visiting our website.
This opportunity to make an impact is precisely what led us to go beyond our usual role of service provider to join forces with Dr. Joanna Newton, MD, Georgia Tech Researchers and Students, and Children’s Healthcare of Atlanta Pediatric Technology Center to address the shortage of PPE for frontline healthcare workers during the pandemic and supply accessible face shield designs to GMP compliant 3D manufacturers.