Lumbar punctures are a procedure performed by neurologists to obtain cerebrospinal fluid from a patient’s back, typically from the L3/L4 spinal canal, and are used for diagnosis and treatment of many neurological diseases and cancers. There are more than 8 million conducted worldwide every year, including 370,000 in the United States.
Currently, doctors feel around the patient’s back to get an understanding of where the bone anatomy is, and make an educated guess of where to insert the needle. This process is called landmarking. [Because the correct landmark is about the size of a dime, clinicians’] standard landmarking practice only has a 30% accuracy in identifying the correct insertion site.
Despite substantial improvements in imaging technology including ultrasound that might seem the means to improve lumbar puncture accuracy for spinal fluid removal, the rudimentary nature of the procedure has not changed for more than two decades. Why? Learning curve time for clinicians, cost increases for all concerned and unacceptable waiting times for imaging tools and technicians when time is of the essence for these patients.
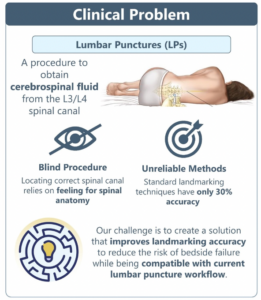 Dr. Rand Ibrahim, a post-doctoral research fellow with the Emory University, challenged 2023 Spring Capstone teams to improve landmarking accuracy and reduce the risk of bedside failure while being compatible with the current lumbar puncture workflow. The five person 2023 Georgia Tech BME Capstone Team dubbed The VerteBrady Bunch developed VerteVision, a device that can be rolled onto a patient’s back at the start of the procedure, and uses pressure to ink areas of the skin with hard bone underneath. “The marked parts of the back serve to highlight patient-specific spinal anatomy such that the doctor can easily and accurately identify the target insertion site for the needle.”
Dr. Rand Ibrahim, a post-doctoral research fellow with the Emory University, challenged 2023 Spring Capstone teams to improve landmarking accuracy and reduce the risk of bedside failure while being compatible with the current lumbar puncture workflow. The five person 2023 Georgia Tech BME Capstone Team dubbed The VerteBrady Bunch developed VerteVision, a device that can be rolled onto a patient’s back at the start of the procedure, and uses pressure to ink areas of the skin with hard bone underneath. “The marked parts of the back serve to highlight patient-specific spinal anatomy such that the doctor can easily and accurately identify the target insertion site for the needle.”
“We spent a significant amount of the time allotted on clinical observation to understand user needs, including shadowing and interviews with those performing the procedure every day,” team member Malak Bayyari said. “We quickly saw just how important improvement in accuracy while fitting seamlessly within the current clinical workflow is for this unmet need.”
“Because there is not currently a predicate device on the market for this need, we had free reign to design what we thought would best solve the unmet need,” Malak continued. “While the solution is not particularly complex, it will likely require a fairly rigorous FDA Class II De Novo regulatory pathway because the device will provide information or guidance on needle placement. If the device does not perform accurately and as intended, it can lead to unacceptable risks, hence the rigorous pathway.”
“Our benchtop testing, which included both proficient and novice clinicians, and a model that used a 3D printed pelvis, lumbar and foam to simulate subcutaneous tissue or fat, showed significant improvements in accuracy and speed of our device compared to the standard of care,” Emma Hagerty said. “But foam doesn’t match the elasticity of subcutaneous fat, nor did our benchtop model substantially match the musculature, ligaments, tendons that would be required for ‘next level’ verification and validation testing. Testing in a cadaveric model needed to be the next step.”
Since 2016, GCMI has proudly supported GCMI Capstone Teams in need of design, development and testing support for their clinician-driven medtech solutions.
“We utilized the Capstone day at T3 Labs to test our device on a human cadaver, which allowed us to verify that the bone markings left by the VerteVision lined up with spinal anatomy of the cadaver revealed by fluoroscopy,” Janhvi Dubey said.
 “Verifying our device’s markings pointed accurately toward patient-specific anatomy is critical to the successful advancement within the regulatory pathway,” Malak said. “The work we did at GCMI and T3 Labs, especially thanks to their team’s support and onsite resources like the fluoroscope and c-arms, accomplished this need and marked a significant moment for the device’s commercialization potential… a real transition from just a prototype to a device with high potential to solve the strong unmet clinical need.”
“Verifying our device’s markings pointed accurately toward patient-specific anatomy is critical to the successful advancement within the regulatory pathway,” Malak said. “The work we did at GCMI and T3 Labs, especially thanks to their team’s support and onsite resources like the fluoroscope and c-arms, accomplished this need and marked a significant moment for the device’s commercialization potential… a real transition from just a prototype to a device with high potential to solve the strong unmet clinical need.”
With the Emory contingent planning to carry forward with provisional patent and licensing efforts, the newly minted alums carry on in their own journeys. Janhvi is joining Abbott’s Professional Development Rotational program and Malak is interning this summer with the industry leader in heart valve technology Edwards Lifesciences with intentions to enroll in the Master of Biomedical Innovation and Development program at Georgia Tech in Fall 2023. Andy starts his pursuit of a Masters Degree in Biomedical Engineering at Tech this fall. Emma is pursuing industry opportunities at the intersection of healthcare and computer science and Jeremy Ortmann is enrolling in the University of Virginia PhD program in biomedical engineering with a focus on regenerative therapies and wound healing.
“Creating promise in our device while experiencing the intensity of the process, getting our feet wet on predicate devices and cadaver testing with solid statistical analysis is incredibly satisfying,” Jeremy said. “All of the moving parts: prototyping, testing, pitching, regulatory, legal and marketing absolutely deepens my respect for medtech startups.”
“Capstone is an inch deep and a mile wide,” Emma said. “Exposure to a fairly comprehensive set of requirements or elements will be especially valuable in future industry endeavors, even as those endeavors may narrow or specialize.”
GCMI is proud to support Georgia Tech BME Capstone projects like Team VerteVision as we have done for dozens of others. We wish the team all the success in their future endeavors and invite you to check out the new collection of GCMI supported Capstone projects here.
