An estimated 10 million Americans suffer from atrial fibrillation (AF). The current medications to treat AF have low success rates and potentially toxic side effects. With preclinical help from T3 Labs and the support of experts within the Atlanta medtech ecosystem, Dr. Rebecca Levit aims to change that.

Dr. Rebecca Levit
Dr. Levit is Assistant Professor of Cardiology at Emory University in Atlanta, GA and adjunct faculty in the Department of Biomedical Engineering at the Georgia Institute of Technology. She is also the Chief Scientific Officer of CorAmi Therapeutics. CorAmi is working in partnership with Emory University to develop a combination hydrogel delivery catheter and therapeutic hydrogel focused on cardiac applications, leading with a treatment for AF. The technology was initially conceptualized in Dr. Levit’s lab at Emory University.
Dr. Levit’s preliminary data collected at T3 Labs shows that the technology is effective in changing the electrical tendencies of the heart: a prospective game-changer for the estimated 10 million Americans and 30 million worldwide living with AF.
The current standard of care for AF rate controlling is antiarrhythmic medications or a highly invasive procedure where the heart is ablated at the areas of AF, the success rate for which is low. Amiodarone is a drug used for treating AF but builds up toxic levels in the liver, lungs, and thyroid. Due to its chemical properties rarely does it end up in the heart where it should be. CorAmi aims to change that.
The team has developed a unique catheter design that allows placement a therapeutics directly in the pericardial space of the heart ensuring efficient delivery and minimizing any harmful effects to surrounding organs; all without requiring open heart surgery. The procedure is minimally invasive, can be done in a cath lab, and only takes about 30 minutes.
Engaging a value-added partner to guide you through the preclinical process
In seeking a preclinical CRO Dr. Levit was referred to T3 Labs. Originally founded as a cardiovascular research institute, T3 has a decade long track-record in preclinical cardiovascular studies and is known for going above and beyond for sponsors.
“At T3 Labs they have the perfect setup for what we needed,” says Dr. Levit. “From the cath lab to fluoroscopy to large animal proficiency, not to mention years of expertise specific to cardiology.
“More so, T3 is a very friendly CRO to work with- the entire staff is excited and interested in our technology. I remember even in testing our first prototype- multiple staff members stuck their head in just to say ‘good luck.’ The customer service is what really sets T3 Labs apart from other CRO’s.”
T3 Labs’ preclinical testing and training program leaders, who played a critical role in the day to management of this study with Dr. Levit, are trained scientists themselves, interested in understanding and participating in the science and methods behind therapeutic options in the cardiothoracic/cardiology space.
“There are so many facets to the process that were foreign to me so the mentorship and teamwork that T3 provided was priceless.”
Designed by cardiologists for cardiologists
“We value T3 Labs because of the top-notch facilities and expertise,” says Dr. Levit. “We were in need of people with practical experience translating our device beyond the initial idea. It was easy to recognize this unmet need, but I did not understand the complexities that it would take to make it a reality: understanding the market, how to derisk a product, how many studies we would need and what documentation is needed for FDA submission and clearance. T3 is a flexible and efficient environment to work in addition to scientific expertise.”
Medtech innovation requires a healthy ecosystem
Dr. Levit engaged regional medtech industry resources early in order to streamline the considerable developmental pathway for a Class III device.
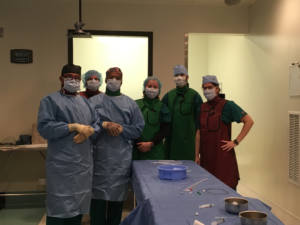
Team testing the CorAmi device in the T3 Labs Cathlab (from L to R) : Dr. Gautam Kumar, Dr. Andres Garcia, Peter Campbell, Juline Deppen, Dr. Jose Garcia, Dr. Rebecca Levit
“The resources here are endless,” she says. “Between meetings with multiple electrophysiologists from Emory and PhDs from Georgia Tech, administrative support and animal study expertise at T3, guidance from the Emory/Georgia Tech Coulter Translational Partnership Fund, working with SEMDA, securing office space at GCMI (not to mention utilizing their fabrication equipment), and funding from multiple organizations, there must be more than 100 people in this area who have touched this product. Needless to say, we are appreciative of the ecosystem!”
What would you tell a physician innovator in your position when you started?
“Conceptually I knew it would take a lot of time. Living through it has given me a better appreciation of how much time is required. Nevertheless, I would still tell myself to go for it! We have learned so much and our advisors believe in the technology. Not just with AF, but for other applications for this delivery method as well. Our advancements with T3 Labs also help drive improvements in our own lab work at Emory.
“Other physician innovators should understand the work it is going to take and that there are some elements that no one can do for you. They can’t write the grant, present the poster or network with people. It takes time and you need to be willing to give that time up. It also takes an ecosystem. This is not at all possible without a healthy medtech development ecosystem. Know that medtech innovation is impossible to do by yourself.”
What’s next for T3 Labs and CorAmi?
“We are completing some important studies and hope to get some promising results in the next few weeks. Our partners and prospective investors are interested in seeing more data,” Dr. Levit says.
As such, the CorAmi team is looking to continue their relationship with T3 Labs engaging in another round of studies supported by funding from the Georgia Research Alliance. CorAmi is also seeking its first round of angel investment to get them to the next round of studies for a Pre IND meeting with the FDA. With the next steps planned, CorAmi is aiming for FDA clearance in two years.
If you are a physician innovator, researcher, or medical device company of any size, and want to bring your new product to life as efficiently as possible, T3 Labs and GCMI can help from concept to cure through preclinical to commercialization. Contact us today. For more information on CorAmi Therapeutics, visit http://www.coramitherapeutics.com/.
