The Transcatheter Cardiovascular Therapeutics (TCT) meeting is the world’s largest educational meeting specializing in interventional cardiovascular medicine. This year, it will take place on October 23-26, 2023 back in San Francisco, California.
“We look forward to connecting with sponsors, clinician innovators and regulatory officials at TCT 2023 to further understand the latest in transcatheter innovations, associated regulatory thinking and how we can better design preclinical studies for transcatheter devices,” GCMI Preclinical Program Manager Ashley Gazaway says.
 “We regularly invest in the TCT annual gathering to ensure we’ve got the capabilities needed to effectively evaluate new TCT technologies, to ensure that we’re up-to-date on regulatory requirements and to learn from regulators directly in order to prepare preclinical reports, especially for GLP preclinical studies, appropriately. More specifically for TCT 2023, we’re especially interested in TAVR, left atrial appendage pumps, atrial clamps, mitral valve replacement technologies, closure devices and procedures to name a few.”
“We regularly invest in the TCT annual gathering to ensure we’ve got the capabilities needed to effectively evaluate new TCT technologies, to ensure that we’re up-to-date on regulatory requirements and to learn from regulators directly in order to prepare preclinical reports, especially for GLP preclinical studies, appropriately. More specifically for TCT 2023, we’re especially interested in TAVR, left atrial appendage pumps, atrial clamps, mitral valve replacement technologies, closure devices and procedures to name a few.”
GCMI is the proving ground for new medical technologies in the United States. Our preclinical arm T3 Labs is an industry-leading preclinical CRO that performs transcatheter cardiovascular device testing and training, among many other therapeutic areas. Founded by cardiologists for cardiologists, T3 Labs’ GLP preclinical program for transcatheter interventional cardiovascular devices includes experience in models of ischemia, transcatheter/interventional implants, telemetry, vascular access etc.
We are a trusted, proven industry-leading preclinical CRO for medical devices and other medical products. We’re an AAALAC accredited facility that conducts feasibility, proof of concept and safety and efficacy GLP medical device testing for studies in any therapeutic area and in small and large models. Since January 2012, we have archived more than 80 GLP studies, including more than three dozen in the last two years alone, for leading medical device manufacturers of which more than 65 products have received regulatory approvals.
“We like to think of ourselves as the gold standard for preclinical work, especially in the cardiovascular realm, and participating in the TCT meeting keeps us on the ‘tip of the spear’ in our practice,” Ashley says.
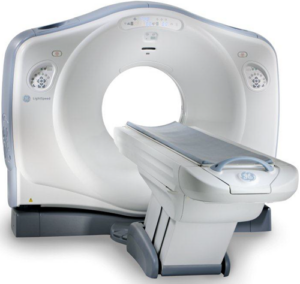 GCMI’s preclinical CRO employs the most appropriate, efficient model and has all the needed equipment for new cardiothoracic technologies like transcatheter therapies including a recently acquired GE LightSpeed VCT 64 Slice CT Scanner with cardiac gating capabilities.
GCMI’s preclinical CRO employs the most appropriate, efficient model and has all the needed equipment for new cardiothoracic technologies like transcatheter therapies including a recently acquired GE LightSpeed VCT 64 Slice CT Scanner with cardiac gating capabilities.
“The ability for these machines to generate 3D images of anatomic structures and organs in a pre-op, post-op or intra-operative scenario, including cardiac and vascular imaging, has improved the safety and efficacy of scores of new medical technologies,” GCMI Director of Scientific Affairs Evan Goldberg said. “Providing immediate on-site access to this technology means our customers can now readily access such capabilities when their preclinical testing protocols require it, and avoid additional costs associated with the same.”
If you would like to get ahead of the game, email Ashley to schedule an onsite meeting at TCT 2023 in advance ashley.gazaway@t3labs.org
Follow GCMI/T3 Labs on LinkedIn and X/Twitter.
In the words of our cardio and TCT-related clients
“At T3 Labs they have the perfect setup for what we needed. From the cath lab to fluoroscopy to large animal proficiency, not to mention years of expertise specific to cardiology.” – Dr. Rebecca Levit, Chief Scientific Officer of CorAmi Therapeutics. Read more.
“We wanted a partner that could help us in developing proper GLP protocols to make sure that we’re doing the research ethically and meeting the safety and quality requirements of the product for future clearance. Another important factor was the execution at the lab itself; understanding the prior training and documentation needed for what we’re trying to accomplish. All of those aspects involve proper process and qualified personnel. T3 checked every box with a glowing gold star. – Vesalio CEO Steve Rybka. Read more.
“Our experience with T3 Labs for the CardioMEMS device created new knowledge and refinements to the device that resulted in a product that was highly mature at the time we started our clinical trials in humans.” Jason White, St. Jude Medical senior director of product development. Read more.
