Mayo Clinic defines cirrhosis thusly: “Cirrhosis is a late stage of scarring (fibrosis) of the liver caused by many forms of liver diseases and conditions, such as hepatitis and chronic alcoholism.
“Each time your liver is injured — whether by disease, excessive alcohol consumption or another cause — it tries to repair itself. In the process, scar tissue forms. As cirrhosis progresses, more and more scar tissue forms, making it difficult for the liver to function (decompensated cirrhosis). Advanced cirrhosis is life-threatening.”
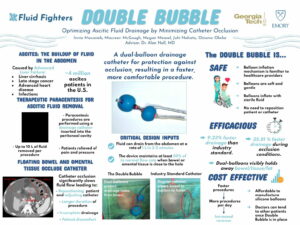
Cirrhosis also causes fluid to build in the abdomen which requires regular, frequent procedures to drain. “While paracenteses are routine procedures that should be painless for the patient, floating bowel or omental tissue can often get suctioned toward the drainage holes and occlude the catheter. Catheter occlusion causes discomfort for the patient and lengthens the duration of the procedure as the healthcare provider must reposition the patient or adjust the catheter to obtain sufficient fluid flow again.” – The Fluid Fighters
When bench testing with synthetic materials just won’t do
 The Fluid Fighters, one of the Georgia Tech Spring 2021 Capstone teams worked, “To provide a solution to catheter occlusion, [through the] the Double Bubble catheter which uses dual-balloon technology to protect drainage holes from bowel or tissue that may approach the catheter. The Double Bubble requires no intervention by the healthcare provider throughout the duration of the procedure, and it uses a balloon inflation mechanism that many healthcare providers are already familiar with, thus allowing it to be easily integrated into today’s procedures.
The Fluid Fighters, one of the Georgia Tech Spring 2021 Capstone teams worked, “To provide a solution to catheter occlusion, [through the] the Double Bubble catheter which uses dual-balloon technology to protect drainage holes from bowel or tissue that may approach the catheter. The Double Bubble requires no intervention by the healthcare provider throughout the duration of the procedure, and it uses a balloon inflation mechanism that many healthcare providers are already familiar with, thus allowing it to be easily integrated into today’s procedures.
The members: Innia Macasieb, Maureen McGough, Megan Measel, Juhi Nahata and Ozioma Okeke. Key advisor: Alan Noll, MD, Internal Medicine Resident, Chief Resident of Quality and Patient Safety, Emory University School of Medicine.
The team needed to test its prototype for draining rates and tissue adherence or occlusion avoidance. GCMI’s preclinical testing and bioskills training arm T3 Labs provided the facility and resources the team needed to complete the task.
Three of the team’s members, each of whom has an immediate family tie to clinical care, spoke with us about what they learned and what they will carry forward.
“While the results from our cadaveric testing at T3 Labs were not what we expected, we did find the technology promising compared to catheters on the market currently used in the procedure with respect to occlusion in the cadaveric model GCMI facilitated,” Megan said. “If we are or were to proceed, we would likely need an external manufacturing resource to produce test articles at a smaller scale than our prototype that can still meet pressurization tolerances in a hospital or procedural environment.”
What they learned and what others can learn from it: medtech innovation is hard, but noble. Keep your focus firmly on the user and patient needs early.
“Simulating human tissue in bench testing with plastic bags is vastly different from testing in real human tissue,” Innia said.
“Our work at GCMI and T3 Labs made the whole experience seem much more real,” Megan said. “As an engineer, you can look at the math, work the engineering problem and develop a potential solution. But it is easy to lose the personal connection with patients on how much it matters, how it will be implemented. How it actually works.”
“We had a good idea about what the device would look like,” Juhi said. “But putting it to use in a near-clinical environment to determine its efficacy and human compatibility is something else entirely. The design and development process for new medical technologies is not for the faint of heart.
“Understand the problem, the patient and clinician needs and orient towards those, not what you think is the best option. Innovation does not have to be groundbreaking or crazy to have a meaningful impact on peoples’ lives. Improving an existing technology can be just as important as groundbreaking or breakthrough technologies.”
“When it comes to device development, focus on the user needs in the earliest stages of your work,” Innia continued. “Without multiple perspectives to define the unmet needs and user requirements, we would not have solved for that need in a way that shows promise for the technology.”
More broadly speaking perseverance is your best friend, Megan said. “Your great idea will not go as planned in testing. Pick it up and try again. You will experience that a lot in biomedical engineering. Eventually what you will make will be helpful.
“Take full advantage of the resources available to you. Use any connections you have to acquire the perspectives and needs required. They can pour invaluable wisdom into your work,” Innia said.
“Telling prospective employers I’ve done this work, this testing, I’ve made a working prototype is huge.” Megan said. “Being this young with this experience and a Georgia Tech degree in Biomedical Engineering puts me in a wonderful place for the future.”
You can watch the team’s 2021 Spring Capstone Design Expo presentation here.
GCMI congratulates the team on its important work and wishes each member all imaginable success in their future endeavors.
GCMI’s mission is to bring new medical technologies to market that improve quality-based outcomes and delivery of care for patients worldwide. This certainly includes supporting development of medical technologies born in our backyard from faculty, researchers and students with whom we share our institutional affiliation. We congratulate the 2021 Spring Capstone teams and eagerly anticipate what the Fall 2021 teams have to show us later this year.
