“We needed to conduct the study perfectly to prove to the FDA that our technology really was safe and effective; we learned that we needed to engage the right partners to do that.” – Innoblative Co-Founder and CEO Tyler Wanke
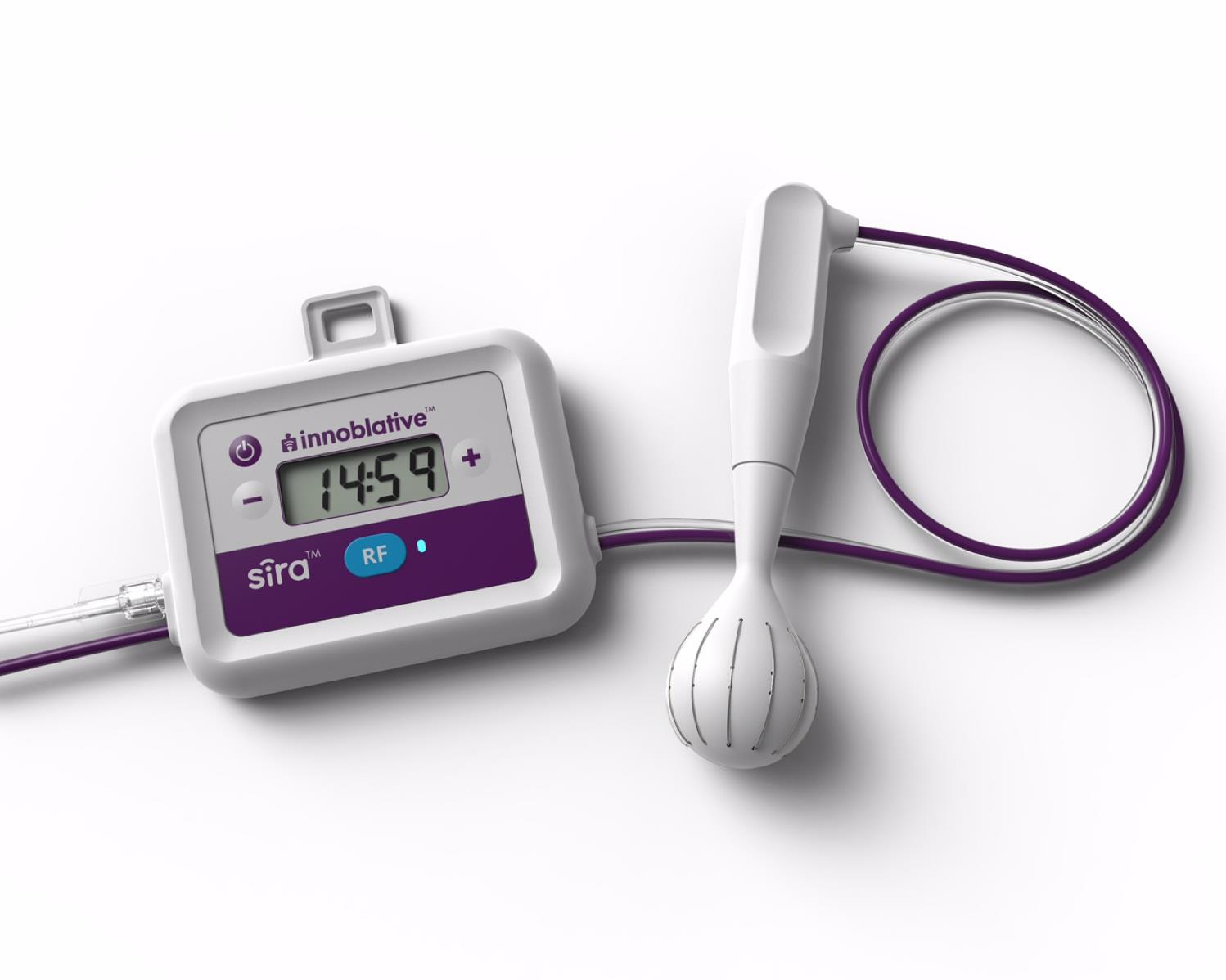
![]() Innoblative seeks to change the game for intraoperative coagulation and ablation of soft tissue via radiofrequency. Its SIRA device is a single-use, disposable applicator used in conjunction with a radiofrequency electrosurgical generator to effectively ablate target tissue. A unique, elegantly-designed 4cm diameter spherical electrode creates a wide ablation zone, covering a larger surface area per ablation compared to devices currently available. The device achieved FDA 510K clearance in April 2019.
Innoblative seeks to change the game for intraoperative coagulation and ablation of soft tissue via radiofrequency. Its SIRA device is a single-use, disposable applicator used in conjunction with a radiofrequency electrosurgical generator to effectively ablate target tissue. A unique, elegantly-designed 4cm diameter spherical electrode creates a wide ablation zone, covering a larger surface area per ablation compared to devices currently available. The device achieved FDA 510K clearance in April 2019.
Though, there were moments when it looked like that was far, far away from happening.

Tyler Wanke, Innoblative Co-Founder and CEO
Innoblative’s early GLP preclinical studies raised serious concerns when a request for additional information from the regulatory body created company-wide panic. Highly confident in the technology, the company attributed this disturbing development to insufficient attention to detail in their prior preclinical study.
“From that point, it was all hands on deck. We needed the very best preclinical partners we could find to execute the study perfectly, among the highest compliance standards, protocols and extremely high levels of attention to detail.”
A former-FDA regulatory consultant referred Innoblative to GCMI/T3 Labs as one of the top labs of its kind in the country. The result: a 510K submission that returned zero questions and a regulatory clearance within 1.5 months.
How did T3 Labs meet those needs?
Ability to expedite
“When you’re pulling clinicians out of their practices for preclinical work, the study must be ready to go when they are,” Tyler said. “One single problem can trigger an avalanche of problems capable of burying a young company like ours. For example, having an anatomically unsuitable model can have a massive cascading effect on the results of the preclinical study. When this happened during our study, T3 quickly enrolled replacement preclinical models within timelines tight enough to avert any programmatic delays and remained not only respectful of our surgeons’ schedules, but within compliance for GDP and GLP.”
Access to multiple surgical oncologists and board certified veterinarians on staff
“We knew we would need to recruit specialists in surgical oncology, at least in part because the FDA wanted multiple physicians exposed to the device,” Tyler said. “The Atlanta medtech ecosystem is full of great hospitals and great surgeons. GCMI and T3 did a tremendous job with coordination, scheduling, flexibility and logistics to ensure those doctors felt comfortable and respected in a familiar environment and convenient location.
“This was a make-or-break moment for us. Having credentialed professionals managing the study, board certified veterinarians on staff and a team who cared about the project as if it was their own, is a distinguishing feature for GCMI/T3 Labs.”
Comprehensive resources, comprehensive results
“We set a very aggressive set of timelines and endpoints,” Tyler said. “The access to ecosystem resources, the staff qualifications, regimens on veterinary care and all-team coordination were paramount to achieving a GLP 510K submission that returned with zero questions asked in a very short timeframe.”
How it showed and what it meant
“The GCMI/T3 Labs team helped us learn, were always well prepared and were intimately concerned with the project,” Tyler said. “It is more cost effective for companies and investors to get it right the first time. Go slightly slower when selecting the right set of studies, the right data sets, the right partner qualifications, and the odds on meeting your endpoint on or ahead of schedule increases.”
We are eager to see Innoblative reach its next milestones for in-vivo clinical studies and commercialization.
About
GCMI is the U.S. proving ground for global medical innovation. Our mission is to accelerate the speed and capital efficiency with which new medical devices and products are brought to market and patient care.
Our preclinical testing arm T3 Labs is an AAALAC accredited, GLP compliant facility that conducts feasibility, proof of concept and safety testing for studies in any therapeutic area and in small and large models. Our staff of biomedical engineers and veterinarians understands our customers’ science and are prepared to support sponsors at multiple inflection points on their pathway from concept to commercialization.
If you would like to learn more about GCMI and T3 Labs email info@t3labs.org