In 2018, GCMI chronicled one Georgia Tech Capstone team’s effort to end complications from epidural misplacement.
Fast forward more than three years and the promising product dubbed Neuraline has evolved to become a new medtech startup: Ethos Medical. The Ethos team leveraged the supporting elements and mentors within the Georgia Tech CREATE-X program—with assistance from GCMI and T3 Labs—to advance development of its needle guidance system.
“Capstone was the springboard,” Ethos Co-Founder, CEO, and Georgia Tech Engineering Alumnus Cassidy Wang told us recently. “Every experience since then has been part of a continuing lesson on the aspects of commercialization beyond developing something that works.

Ethos Medical Co-Founder and CEO Cassidy Wang
“In 2018, our CREATE-X experience dealt with the uncertainty surrounding our initial product,” Cassidy said. “We leaned on mentors and were active in customer discovery, especially with clinicians and hospital administrators. We kept asking ourselves, ‘Are we doing things the right way? Then how do we proceed after identifying the real problem?’”
The team learned that while they were indeed addressing an important, unmet clinical need, they needed to ask a different question in order for their technology to have commercial and clinical impact at sufficient scale.
“When we asked clinicians what their ‘silver bullet’ for spinal access procedures would be—what they most needed or wanted, no matter how realistic or fantastical—every single person said, ‘X-ray vision,’” Cassidy told us.
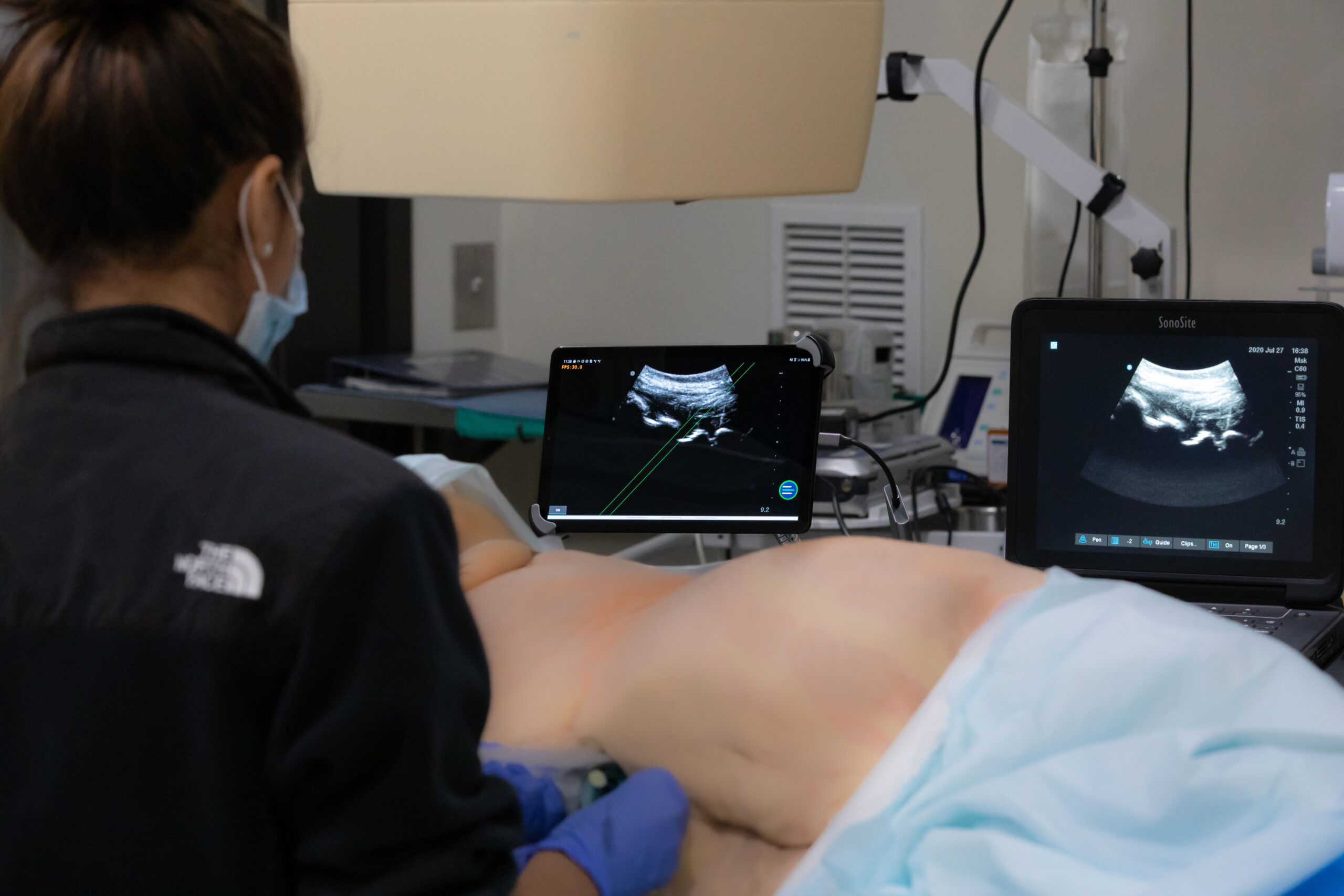
With this insight in hand, the team created a guidance system compatible with the Lumify, an existing handheld ultrasound system produced by Philips.
Beyond the Unmet Need: Successful Medtech Commercialization Must Meet All of a Customer’s Needs
During the team’s 2019 CREATE-X Startup Launch accelerator program, they discovered market-related issues that may well have sunk a young company if they had not identified them early.
While the Lumify-compatible guidance technology performed well, customer interviews revealed logistic and financial issues that the team were unlikely to overcome. Many hospitals already held contracts with manufacturers other than Philips and simply were not willing or able to add ‘adjacent,’ redundant ultrasound systems at almost any cost, no matter how much or how little.
“Most of the clinicians we spoke to were excited about our guidance technology, but almost every one of them would also ask at the end of our hands-on demo, ‘Is it available for that ultrasound machine in the corner over there?’” The team realized that they needed to integrate real-time guidance into machines already at the bedside.
“Without the ability to rely on consistent feedback from our mentors, we would have struggled to interpret the torrent of information we received from clinicians and administrators,” Cassidy said. “That was a huge benefit that enabled us to shift course in order to most accurately address exactly what the market needed our technology to do, and how it needed to do it.”

Ethos Medical Co-Founder and Chief Technology Officer Lucas Muller
Honing in on the right solution with real commercial potential
With the help of a $225,000 SBIR grant in early 2020, the team set out to develop a more flexible system it calls the Iris. The Iris is based on Ethos’ same core real-time guidance technology, implemented in a system that is universally compatible with any ultrasound on the market. The SBIR grant-funded product development activities included feasibility and preclinical testing at GCMI’s T3 Labs. These tests have directly contributed to the team’s successful completion of a functional Iris prototype. The team conducted a series of four cadaver studies to demonstrate the technology’s efficacy, performance, and ease of use in a real operating room environment with real emergency room practitioners.
“Thanks to guidance from T3 Labs and from our CREATE-X mentors, we refocused on the right questions, solutions, and activities with commercial success and clinical improvement as the ultimate goal,” Cassidy said. “We don’t just want to make something that works. We want to make something that works in a way that people need and want it to work, and something that providers will actually pay for.”
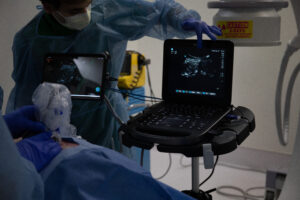
Ethos Medical’s Iris Real Time Guidance Technology
The fourth in the series of preclinical feasibility studies at T3 Labs was the peak of the team’s SBIR project. After a 30-minute training session, the participants, all practicing emergency room physicians or nurse practitioners, were mostly if not entirely proficient with the device. The study generated statistically significant improvements in critical procedure metrics compared to the standard technique.
Cassidy told us that the team has its regulatory strategy in place and, while it polishes last mile design tweaks integrating additional technology and features, it is fast approaching more rigorous verification and validation testing. Manufacturing transfer activities will follow. The team’s target is an FDA 510(k) clearance in late 2022.
Proudly supporting the development and commercialization of new medical technologies
“GCMI and T3 Labs have always been generous and accommodating in every study we have conducted at their facility with their assistance,” Cassidy said. “We are able to rely on their team to get the job done properly and efficiently, even on a moment’s notice. As first-time entrepreneurs, it is inspiring to work in their environment, with their team, equipment and experience including preclinical study protocol design.
“From our Capstone experience and those of the past three years, we have learned where to trust ourselves and where we need to refer to others’ expertise. It has kept us moving in the right direction, even when that path needs to change. Intuition can lead innovators astray if solely based on science or technology. But reliance on intuition can be fatal to a new endeavor or young company. Know your boundaries, but be confident in your abilities.”
The GCMI and T3 Labs team proudly supports Cassidy and the entirety of the Ethos Medical team. We thank them for helping us to demonstrate, at least in part, how we work to fulfill one core element of our mission: to support the development and commercialization of medical technologies including those born from the minds of students, graduates, principal investigators, engineers and academic faculty at Georgia Tech.