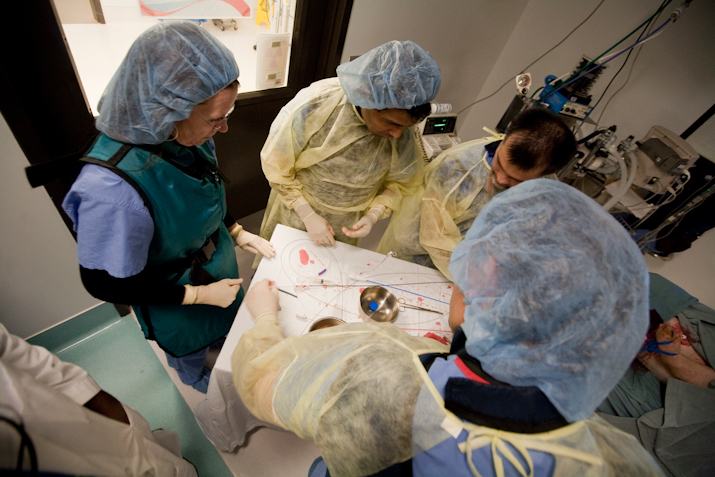
What you need, when you need it
- Lap towers and insufflators
- ECHO including ICE, TTE and TEE
- C-arms
- MRI and CT
- Surgical instrumentation
- Cadaveric and in-vivo models

From concept to cure: the full circle of new medical product development
Our expertise does not end at preclinical testing. We have a comprehensive understanding of Stage I – Research and Development, via our affiliation with GCMI (Global Center for Medical Innovation), the Southeast’s first and only comprehensive medical device innovation center dedicated to accelerating development, building businesses and improving health. GCMI works with startups, clinician innovators, university tech transfer offices and academic researchers to design, engineer, prototype, and facilitate commercialization of a broad range of innovative medical devices.
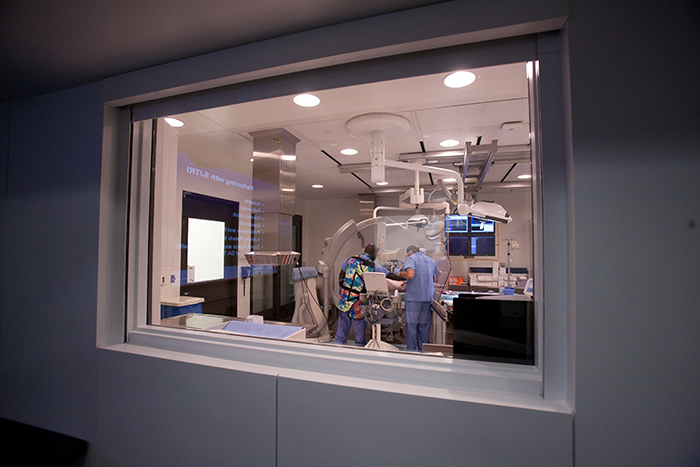
Concierge level service in medical device training
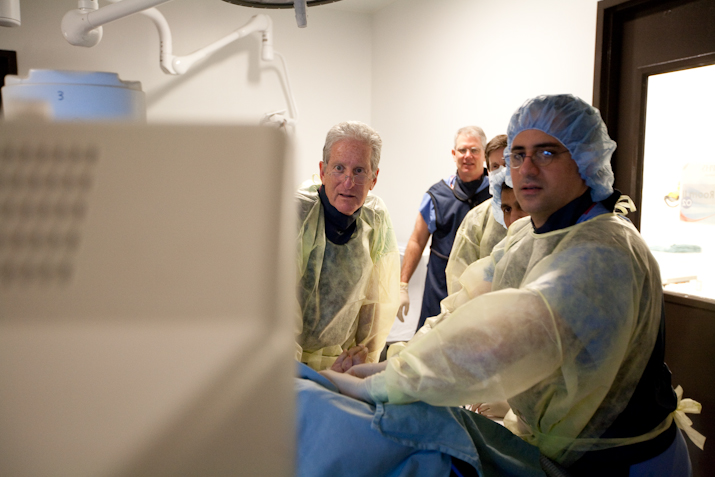
Experience
- Colon resection and defect repair
- Bariatric surgery
- Robotic surgery
- Wound healing – organ laceration
- Stenting
- Interventional radiology – embolics for liver, kidney
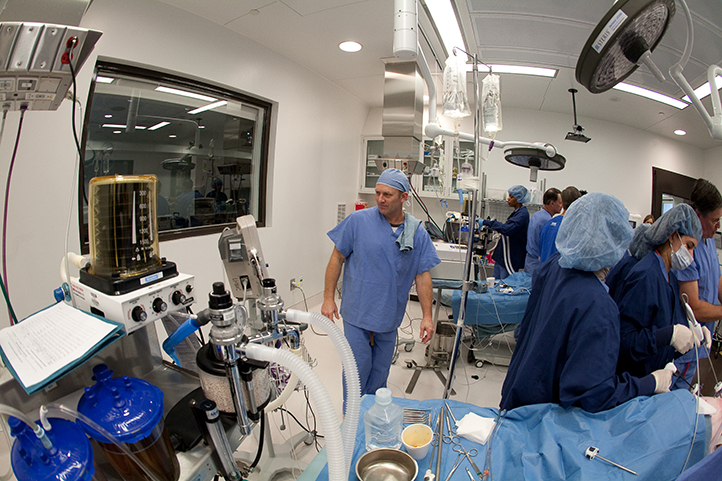
Our program leaders are scientists as well
Our preclinical medical device testing and training team has archived more than 50 GLP studies and more than 30 products have received their FDA approvals. From concept to cure, we help bring your innovation full circle.