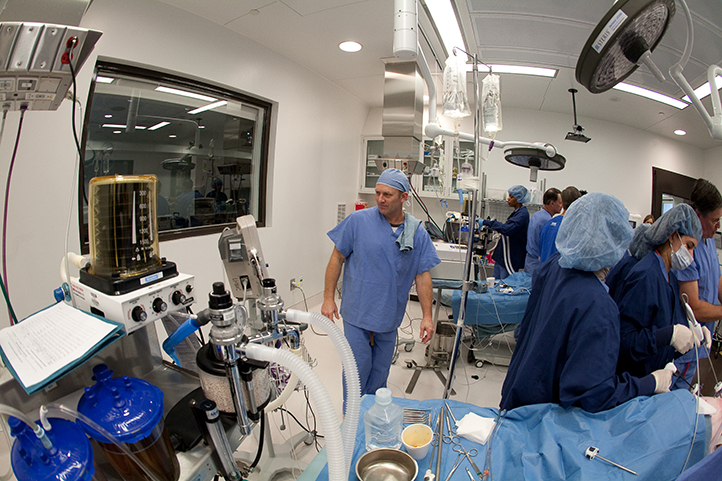
GCMI is committed to providing superior access, service, quality and deliverables in a preclinical CRO. Our facility provides physicians, sales staff, students and allied healthcare professionals what they need and when they need it with the highest quality service designed with your bioskills training needs in mind. Keep reading to see what sets GCMI apart.
WHY GCMI?
Flexibility
We work beyond the bankers hours because we know time is of the essence, deadlines must be met and your innovation is not your surgeons’ sole concern.
Collaborative Accountability
We are collaborative and accountable. We are committed to meeting your needs on your timeline and on your budget.
Quality, Compliance & Accreditation
GCMI is committed to meeting customer needs and regulatory requirements. Our AAALAC accreditation and GLP experience validate these claims.
Our Scientific Acumen
We won’t turn your project away because it’s too hard, or because we haven’t done it before. We want to work with you to address real challenges.
Commitment to Value
Our response to your request for preclinical work may not be the lowest bottom line price, but all preclinical programs and RFPs are not created equal.
Flexibility
As the preclinical CRO industry consolidates, creativity and flexibility suffer. GCMI and our preclinical arm, T3 Labs, work around your needs, around you and your surgeons’ availability, not the other way around. We work beyond the banker’s hours because we know time is of the essence, deadlines must be met and your innovation is not your surgeons’ sole concern.
If your surgeons have just three days to complete their work, we’re there! Our industry-leading preclinical CRO is fully committed to delivering what you need, when you need it. On your schedule, within your budget.
“T3 Labs has the track record, facilities, expertise, and flexibility to help innovators reach FDA clearance on time and on budget.”
Dr. Tom Barrows, President & CSO, Cell Constructs

“Preclinical work is always filled with unexpected outcomes – some good, some bad. Having a trusted preclinical partner that has the experience, expertise, and flexibility to work with your specific product, team, timeline and budget is of the utmost importance.T3’s recommendation for the model shift based on their experience and expertise was much more practical for this application based on our device’s capabilities and needs. I am convinced this recommendation has accelerated our product refinement testing and preclinical trials saving ALung time and money at every step along the way.”
Jeremy Kimmel, PhD, Director New Technology, ALung Technologies

“Having an anatomically unsuitable model can have a massive cascading effect on the results of the preclinical study. When this happened during our study, T3 quickly enrolled replacement preclinical models within timelines tight enough to avert any programmatic delays and remained not only respectful of our surgeons’ schedules, but within compliance for GDP and GLP.”
Tyler Wanke, Co-Founder and CEO, Innoblative

Collaborative Accountability
In this era of consolidation, the dwindling number of preclinical CROs often aren’t interested in developing new models or venturing outside of their well established protocols.
Why choose GCMI for your preclinical testing needs? We are collaborative and accountable. We are committed to meeting your needs on your timeline and on your budget.
We won’t turn your project away because it’s too hard, or because we haven’t done it before.
We stand by our work. And if a regulatory body comes back with questions, we’re happy to work with them quickly so as to not hold up the application. However, it is much more common for regulatory submissions supported by data generated at T3 Labs to return zero questions.
Another promise: we will never mislead you on price.
“Every T3 Labs scientist exhibits exemplary levels of professionalism when it comes to interacting with the physicians and associated team members we bring in to conduct our preclinical tests. T3 Labs has been a true partner throughout this entire process.”
Steve Rybka, Vesalio CEO

Quality, Compliance & Accreditation
At GCMI, we strive to provide the highest quality data for our preclinical sponsors and customers.
GCMI is committed to working with innovators to test medical technologies and therapeutics that consistently meet customer needs and regulatory requirements. We maintain Quality Management Systems to ensure compliance and ongoing quality of our deliverables, products and services.
Our AAALAC accreditation and GLP experience validate these claims. But you don’t have to take our word for it.
You don’t have to settle for cookie-cutter preclinical services and programs. You can demand high-quality, accredited preclinical support on your timeline within your budget.
“We needed to conduct the study perfectly to prove to the FDA that our technology really was safe and effective; we learned that we needed to engage the right partners to do that. Having credentialed professionals managing the study, board-certified veterinarians on staff and a team who cared about the project as if it was their own, is a distinguishing feature for GCMI/T3 Labs.”
Tyler Wanke, Co-Founder and CEO, Innoblative

Our Scientific Acumen
At GCMI and our preclinical testing arm T3 Labs, we take great pride in our scientific acumen. Afterall, the organization was conceived and designed by translational researchers for medical product developers. Thirst for science: it’s in our DNA.
We won’t turn your project away because it’s too hard, or because we haven’t done it before. Other preclinical CROs often aren’t interested in developing new models or venturing outside of their well established protocols. We want to work with you on things that are hard or haven’t been done before.
In one example among many, T3 Program Director Irena Brants, along with Alizee Pathology, published the first preclinical testing documentation that a progressive regenerative response occurs as early as 7 days after renal denervation in “Circulation: Cardiovascular Interventions.”
“Having credentialed professionals managing the study, board-certified veterinarians on staff and a team who cared about the project as if it was their own, is a distinguishing feature for GCMI/T3 Labs.”
Tyler Wanke, Co-Founder and CEO, Innoblative

Commitment to Value
We know preclinical testing is a substantial commitment of time and treasure for medtech innovators. We also know the most expensive study is the one you have to do twice.
Our response to your request for preclinical work may not be the lowest bottom line price. But please know this: all preclinical programs and RFPs are not created equal. They may or may not include line items that can significantly influence the total fee. Much more goes into the making of a high-quality, value-based preclinical study that is critical, but is not always obvious.
Know this as well, we will never mislead you on price.
What costs should you expect, and what should you watch out for, when planning your next preclinical study? Will you need specialty supplies or drugs? If you budget three hours for a procedure that ultimately takes five will you see additional charges? We invite you to read more about the common costs in preclinical research you should expect, and what you should watch out for, in your next preclinical program.
CONTACT US
If you are evaluating your readiness for preclinical or are ready to put a program and protocol together, let’s talk! Fill out the form below to get in touch with us, or email Evan Goldberg directly at evan.goldberg@t3labs.org to start the conversation now.