YOUNG THERAPEUTICS, LLC
Successfully Guiding Medical Innovation
GCMI is proud to work in over 11 therapeutic areas and a wide array of medical products (Class I, II and III) including drugs and biologics. Listed below are some of the projects that we have had the pleasure to be a part of and further information on what roles we played in its development and to what result. Please contact us today if you have any questions or are a physician innovator or engineer with an idea for a new device.
Please note, this page is a work-in-progress with more examples of our work to come.

Young Therapeutics, LLC
The Unmet Need
During events such as myocardial infarction (heart attack) and surgical procedures involving angioplasty, bypass surgery, or heart transplantation, reactive oxygen species (ROS) can be generated, which cause organ and blood vessel damage. Our novel treatment is aimed to prevent the generation of ROS from all four of these sources. ROS generated from these sources is known to extend the infarct size after myocardial infarction and is a key component of reperfusion injury.
The Process
GCMI and our preclinical arm, T3 Labs, helped refine Lindon’s SBIR Phase I grant application with a tweak to study protocols in order to more closely mirror clinical application. The protocol, originally drafted as IV to jugular, was changed to intra-arterial, based on early surgical input.
“The surgeons told us they are most likely to deliver this therapy straight to the artery,” Lindon said. “Adopting a protocol that more closely mirrors the actual clinical application in humans, was a high value recommendation brought forth in advance of the in-vivo preclinical work. Working with the great team at T3 Labs has dramatically improved the timeline to test the novel molecules in the clinic, most notably to heart attack patients receiving percutaneous coronary intervention (PCI).”
The Results
“We have completed testing of the first test agent and the results indicate a robust reduction in infarct size (i.e. 70% reduction) in the test compound vs.the control compound group accompanied with significant improvement in post-MI cardiac function.”
Read more about Dr. Young’s experience with T3 Labs here.
JACKSON MEDICAL
GloShield™

GloShield™ - Jackson Medical
The Unmet Need
Overheating lighting instruments coupled with human error are a leading cause of intra-operative fires and patient burns. The healthcare system refers to these as “never events.” Sadly, data indicates that they occur more than twice a day.
The Process
 Jackson Medical Co-founders James Rains and Kamil Makhnejia were joined by Dr. Spencer Kozinn, a local Atlanta urologist, to create GloShield.™ In 2017, the GCMI Phase Zero Program paved a pathway for Jackson Medical to further develop the innovative technology that reduces dangerous thermal conditions in the operating room.
Jackson Medical Co-founders James Rains and Kamil Makhnejia were joined by Dr. Spencer Kozinn, a local Atlanta urologist, to create GloShield.™ In 2017, the GCMI Phase Zero Program paved a pathway for Jackson Medical to further develop the innovative technology that reduces dangerous thermal conditions in the operating room.
“GCMI helped to characterize our use need and the thermal environment, then prototyped solutions that met the more refined specifications,” says Rains. “We knew that we had an innovative concept and that there is nothing like GloShield currently on the market, but needed to ensure that we were taking the correct steps that would help us reach commercialization in a timely and cost-efficient manner.”
For Jackson Medical, GCMI’s Phase Zero program developed intellectual properties and a functioning prototype as well as guidance on next steps in the pathway from concept to commercialization.
The Results
 GloShield is a reliable solution that reduces the risk of fires and burns in the operating room attributed to fiber-optic light cables. With seamless implementation onto existing cables, GloShield limits “never events” and prevents thermal injury.
GloShield is a reliable solution that reduces the risk of fires and burns in the operating room attributed to fiber-optic light cables. With seamless implementation onto existing cables, GloShield limits “never events” and prevents thermal injury.
SALDANA RESEARCH GROUP & GEORGIA TECH
PPE Face Shields

PPE Face Shields - Saldana Research Group & Georgia Tech
Unmet Need
In early 2020, U.S. frontline healthcare workers were in desperate need of personal protective equipment (PPE) as Covid-19 began to ravage the country and its healthcare system.
By March 13, Christopher Saldana, Ph.D., associate professor of manufacturing at Georgia Tech and his mechanical engineering students and colleagues were already working on challenges and solutions for PPE for frontline healthcare workers.
Chris’ team needed a scalable, final design capable of transitioning to large scale manufacturing to meet the end users’ urgent needs for PPE face shields.
Our Process
Thanks to the close knit medtech innovation ecosystem in Georgia, Sherry Farrugia and Joanna Newton, MD, connected Christopher and his team with GCMI.
The GCMI / Saldana Research Group / Georgia Tech team went into ‘all hands on deck’ startup mode to rapidly design, prototype and iterate. Chris’ team used their fabrication facility to produce the face shield prototypes. GCMI documented clinical feedback, functionality and traceable document specificity. GCMI helped the team understand the use case in the hospital setting as well as requirements for large scale manufacturing. We helped guide Saldana’s team’s prototyping because manufacturers need specific details on manufacturing allowances and tolerances to deliver the product the frontline clinicians need.
Results
The collaboration produced the final designs including documentation and regulation considerations to enable manufacturing partners to produce the face shields and begin distribution to the front line providers and hospitals.
To date, more than 2 million PPE face shields have been delivered to front line healthcare workers across the country. The team made the designs freely available to all, so others like KIA have been manufacturing face shields using the Saldana design and GCMI specifications for use under EUA. The project earned a Georgia Bio 2021 Golden Helix Award for Innovation.

Face Shield Prototype – Rigid
ALUNG TECHNOLOGIES, INC.
Hemolung RAS

Hemolung RAS - ALung Technologies, Inc.
Unmet Need
COPD affects 30 million Americans and is the third leading cause of death in the United States behind cancer and heart disease.
Acute exacerbations, defined as a sudden worsening of COPD symptoms, are a major cause of morbidity and mortality in COPD patients. For patients with severe exacerbations, high levels of carbon dioxide can result in respiratory failure and the need for intubation and mechanical ventilation as life saving measures. Unfortunately, mechanical ventilation is associated with many side effects, and in-hospital mortality remains as high as 30%.
Extracorporeal CO2 removal (ECCO2R) therapy with the Hemolung Respiratory Assist System (RAS) allows carbon dioxide to be removed from the blood independently of the lungs with the aim of facilitating the avoidance or reduction of intubation and invasive mechanical ventilation.
Early, non-GLP studies for the Hemolung RAS were performed in an academic setting. The institutional administrative requirements for these studies, including IACUC, did not move swiftly enough for ALung. As a small medical device company, it needed a CRO with great flexibility and responsiveness in order to maximize limited resources and accelerate the timeline associated with preclinical trials. It also needed assurance of confidentiality.
Our Process
The relationship between GCMI and ALung began back in 2013 when the company chose to partner with T3 Labs for preclinical studies supporting U.S. regulatory approval for the Hemolung RAS. ALung later selected T3 Labs as the GLP preclinical CRO partner in support of their IDE application for the Hemolung RAS in March 2017.
 “Preclinical work is always filled with unexpected outcomes – some good, some bad. Having a trusted preclinical partner that has the experience, expertise, and flexibility to work with your specific product, team, timeline and budget is of the utmost importance,” ALung Vice President of New Technology, Jeremy Kimmel, PhD, told us.
“Preclinical work is always filled with unexpected outcomes – some good, some bad. Having a trusted preclinical partner that has the experience, expertise, and flexibility to work with your specific product, team, timeline and budget is of the utmost importance,” ALung Vice President of New Technology, Jeremy Kimmel, PhD, told us.
“Having a reliable animal model is one of the most important things to have under your belt before going to the FDA,” Kimmel said. “T3’s recommendation for the model shift based on their experience and expertise was much more practical for this application based on our device’s capabilities and needs. I am convinced this recommendation has accelerated our product refinement testing and preclinical trials saving ALung time and money at every step along the way.”
Results
ALung received IDE approval for its VENT-AVOID trial from the FDA in September 2017.
ALung further received FDA Emergency Use Authorization (EUA) for their Hemolung® Respiratory Assist System (RAS) for Covid-19 treatment.
“T3 sets a high bar for both technological capability, customer service, flexibility and collaboration,” Kimmel said. “We are thrilled with T3’s work and plan to continue to expand on our partnership for the foreseeable future.”
CORAMI THERAPEUTICS
Hydrogel and Delivery Device for Atrial Fibrillation

Corami Therapeutics
The Unmet Need
An estimated 10 million Americans suffer from atrial fibrillation (AF). The current medications to treat AF have low success rates and potentially toxic side effects. With preclinical help from T3 Labs and the support of experts within the Atlanta medtech ecosystem, Dr. Rebecca Levit aims to change that.
The Process

Dr. Rebecca Levit
Dr. Levit is Assistant Professor of Cardiology at Emory University in Atlanta, GA and adjunct faculty in the Department of Biomedical Engineering at the Georgia Institute of Technology. She is also the Chief Scientific Officer of CorAmi Therapeutics. CorAmi is working in partnership with Emory University to develop a combination hydrogel delivery catheter and therapeutic hydrogel focused on cardiac applications, leading with a treatment for AF. The technology was initially conceptualized in Dr. Levit’s lab at Emory University.
Dr. Levit’s preliminary data collected at T3 Labs dating to 2017 shows that the technology is effective in changing the electrical tendencies of the heart: a prospective game-changer for the estimated 10 million Americans and 30 million worldwide living with AF.
“We value T3 Labs because of the top-notch facilities and expertise,” says Dr. Levit. “We were in need of people with practical experience translating our device beyond the initial idea. It was easy to recognize this unmet need, but I did not understand the complexities that it would take to make it a reality: understanding the market, how to de-risk a product, how many studies we would need and what documentation is needed for FDA submission and clearance. T3 is a flexible and efficient environment to work in, in addition to the team’s scientific expertise.”
“There are so many facets to the process that were foreign to me so the mentorship and teamwork that T3 provided was priceless.”
The Results 
Corami was awarded SBIR phase 1 funding in 2019 “to adapt our minimally invasive delivery device designed for use in the cardiac cath lab to a surgical device to delivery amiodarone gel after open heart surgery.”
Read more about their experience with T3 Labs here.
ST. JUDE MEDICAL
CardioMEMS HF System

St. Jude Medical - CardioMEMS HF System
The Unmet Need
- 1.4 million patients in the U.S. have New York Heart Association (NYHA) Class III heart failure
- Historically these patients account for nearly half of all heart failure hospitalizations.
- According to the American Heart Association, the estimated direct and indirect cost of heart failure in the United States for 2012 was $31 billion, and that number is expected to more than double by 2030.
The CardioMEMS HF sensor is the first of its kind implantable wireless device implanted in the pulmonary artery to monitor pulmonary artery pressures.
The Process
“During our preclinical work with T3, the concept for the sensor was refined from being attached to the right ventricular septum with an anchor coil to one that is placed in the distal pulmonary artery passively anchored by wire loops. Iterative refinement resulted in an optimized configuration for ease of use during the implant procedure and long term safety. T3 labs provided the ideal environment to identify the optimal configuration prior to first clinical use,” St. Jude Medical Senior Director of Product Development Jason White says.
“There are significant portions of preclinical and GLP requirements that the T3 Labs team knows that we don’t: not only in their knowledge of study subjects, and administration of a GLP study, but also follow up tests and biometrics that are commonly included in regulatory submissions. The CardioMEMS team includes T3 Labs in our new device protocols because of their industry leading knowledge of regulatory requirements for preclinical and beyond.”
The Results
- After filing the IDE (Investigational Device Exemption) with FDA which included data collected from T3 Labs, CardioMEMS received its letter of approval to begin clinical trials in humans.
- After submitting its premarket approval application to the FDA, CardioMEMS received its letter of approval to begin commercial distribution on May 28, 2014.
- The CardioMEMS HF sensor(1) is the first of its kind implantable wireless device implanted in the pulmonary artery to monitor pulmonary artery pressures.
- Following a six-year, $100 million development process, St. Jude Medical completed its acquisition of CardioMEMS for a total of $435 million.
“Our experience with T3 Labs for the CardioMEMS device created new knowledge and refinements to the device that resulted in a product that was highly mature at the time we started our clinical trials in humans. During our preclinical work with T3, the concept for the sensor was refined from being attached to the right ventricular septum with an anchor coil to one that is placed in the distal pulmonary artery passively anchored by wire loops. Iterative refinement resulted in an optimized configuration for ease of use during the implant procedure and long term safety. T3 labs provided the ideal environment to identify the optimal configuration prior to first clinical use,” White says.
Read more about their experience with T3 Labs here.
VESALIO
NeVa
NeVa™ - Vesalio
The Unmet Need
- Previously, IV medications would be given to stroke patients in the hopes they would dissolve or break down clots.
- Current technologies are not effective in removing organized or firm thrombus, which can add to the potential creation of clot fragments that migrate to downstream vessels and often require multiple attempts to remove the clot.
- As the standard of care has evolved to interventional procedures, a need arose for a new device specifically designed to capture the clot in the device and reduce the number of passes needed to effectively recanalize the blocked vessel.
Vesalio’s revascularization device, NeVa, is intended for retrieving clots in the cerebral vasculature to minimize the damage caused by stroke.
The Process

Vesalio CEO Steve Rybka
Vesalio has partnered with GCMI and T3 Labs through multiple GLP and Non-GLP preclinical studies since 2016 to develop their family of devices in an effort to reduce the amount of “passes” or attempts required to remove a variety of clot types while reducing fragmentation that can result in clots migrating to more difficult or impossible to reach areas of the brain. Their NeVa device creates a means to interact with the clot directly and improve the ability to get even the most difficult calcified clots inside the device’s basket, or nitinol tube. With its unique design and with the NeVa Drop Zone Retrieval Technique™ that they have been developing with their leading users, Vesalio believes they have a real shot at improving first pass success in emergency stroke intervention.
“T3 Labs has been a true partner throughout this entire process,” shared Vesalio CEO, Steve Rybka. “We understand and are appreciative of the value they bring to development, quality and regulatory processes. T3 checked every box with a glowing gold star. It’s a complex relationship that’s very different from our other outsourced partners that we work with.”
The Results
- As a direct result of GLP preclinical studies performed at T3 Labs, Vesalio received a CE mark in October 2017.
- Vesalio obtained an FDA 510k clearance and its 4th CE approval in January of 2021.
“We have not once veered from our relationship with T3 Labs and have no intentions of doing so,” Rybka stated. “I wish I could bring the T3 staff to Europe for research programs that must be done there. The T3 staff handle everything required with the utmost professionalism and consideration.”
Read more about their partnership with T3 Labs here.
CELL CONSTRUCTS
ProgenaMatrix™

ProgenaMatrix™ - Cell Constructs
The Problem
Cell Constructs is a regenerative healthcare company specializing in wound care healing that was in the process of developing their first product, ProgenaMatrix™, an active wound care solution utilizing human keratin. The company knew that, while not always required for FDA approval, completing preclinical studies can be advantageous for proof of concept, raising capital, applying for reimbursement, and planning for future generations of the medical product. Thus, they began searching for the perfect preclinical partner.
The Solution
Dr. Tom Barrows, President & Chief Science Officer of Cell Constructs and industry veteran, entrusted T3 Labs as Cell Construct’s preclinical partner when the time came to select a CRO due to our track record, facilities, expertise, and flexibility when helping innovators reach FDA clearance on time and on budget. Atlanta’s rich medtech ecosystem and network of medical product and healthcare professionals that work for and with T3 Labs played a huge role in Barrows’ choice.
“We prefer to do business within the Atlanta medtech ecosystem as much as possible so when choosing a CRO, it was nice to have a high quality CRO in our backyard,” Barrows explained. “We often use cultured cells that are important to have on ice so it is a huge deal that we [were] able to drive them down the road and not have to ship them in special packaging. Doing studies with T3 is convenient – some people don’t know that it is even possible to do large animal studies in Atlanta.”
The Results
Cell Constructs received 510(k) clearance from the US FDA for ProgenaMatrix, an active wound matrix manufactured from human keratin and numerous other proteins, on January 17, 2019.
“The [T3] team allowed us to make changes as the process went on, use our own techs – who they welcomed with open arms – and to stay and complete a study that was taking much longer than expected,” Barrows added. “We cannot think of a better CRO to work with and as we continue to add new products to the pipeline, we will certainly utilize T3 Labs for preclinical studies.”
DR. SCOTT HOLLISTER, CHILDRENS HEALTHCARE OF ATLANTA
First 3D Printed Pediatric Tracheal Implant

ProgenaMatrix™ - Cell Constructs
The Problem
A 7-month-old boy battling congenital heart disease and tracheo-bronchomalacia, a condition that causes severe life-threatening airway obstruction. During his six-month inpatient stay in the Pediatric Intensive Care Unit, he experienced frequent episodes of airway collapse that could not be corrected by typical surgery protocols. The clinical team proposed surgically inserting an experimental 3D-printed tracheal splint to open his airways and expand the trachea and bronchus.
The Solution
Scott Hollister, Ph.D., who holds the Patsy and Alan Dorris Endowed Chair in Pediatric Technology, a joint initiative supported by Georgia Tech and Children’s Healthcare of Atlanta, developed the process for creating the tracheal splint using 3D printing technology at University of Michigan C.S. Mott Children’s Hospital prior to joining Georgia Tech. The Children’s procedure was the 15th time a 3D-printed tracheal splint was placed in a pediatric patient.
GCMI provided the business, administrative and quality support necessary to move Dr. Hollister’s technology from research to the clinic. There are always challenges in moving new technology in the lab into products in the market where it can benefit society. Our collaborative approach helps bring diverse groups of stakeholders together towards a common purpose – to help improve the lives of others.
The Results
In a complex 10-hour surgery, Children’s cross-functional team of surgeons successfully placed three 3D-printed splints around the patient’s trachea on the morning of August 17, 2018. The splints will eventually be absorbed into the body, allowing for expansion of the trachea and bronchus.
NFANT LABS
NeoNatal Feeding System
ProgenaMatrix™ - Cell Constructs
The Problem
In the United States, up to 70% of infants born prematurely and 10% of infants born full term have trouble transitioning to breast or bottle feeding on their own; infant feeding complications are one of the leading causes of delay in discharge from the NICU. Furthermore, infants that don’t reach feeding independence are at risk for returning to the NICU.
The Solution
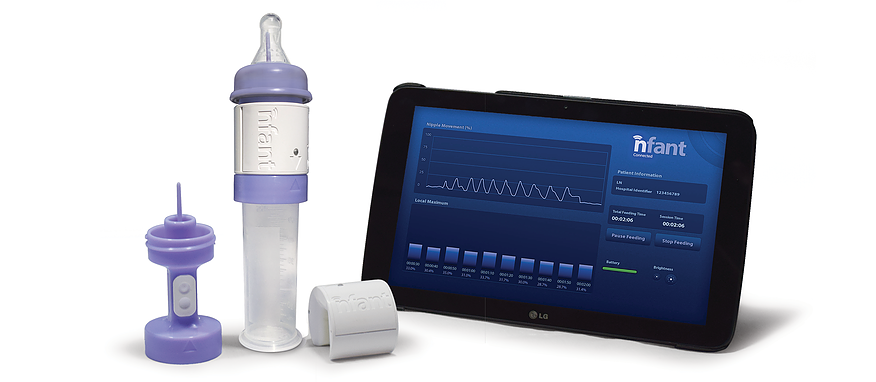
NFANT Labs has created a device that tracks infant progress in feeding. nfant® Feeding Solution is a system that provides data to help clinicians guide premature infants to a safe and healthy transition to independent feeding. The sensor, which connects to standard nipples and bottles, feeds data instantly to the clinician’s tablet, allowing them to see the impact of their therapies and tailor care for each infant.
It all started in a 10 x 10 cubicle at GCMI. ”The decision to move into GCMI’s space provided instant access to the right people and right services for the right price at the right time,” NFANT CEO Lou Malice said.
The Results
Ideation to FDA submission in 10 months. “If we worked with another design and development firm, we wouldn’t have had the flexibility to do what we needed to do in the time frame we set,” Malice said. “The GCMI team always delivered and their sensitivity to time and budget allowed us to take our device from ideation to FDA submission in 10 months.”
INNOBLATIVE
SIRA™ RFA Electrosurgical Device
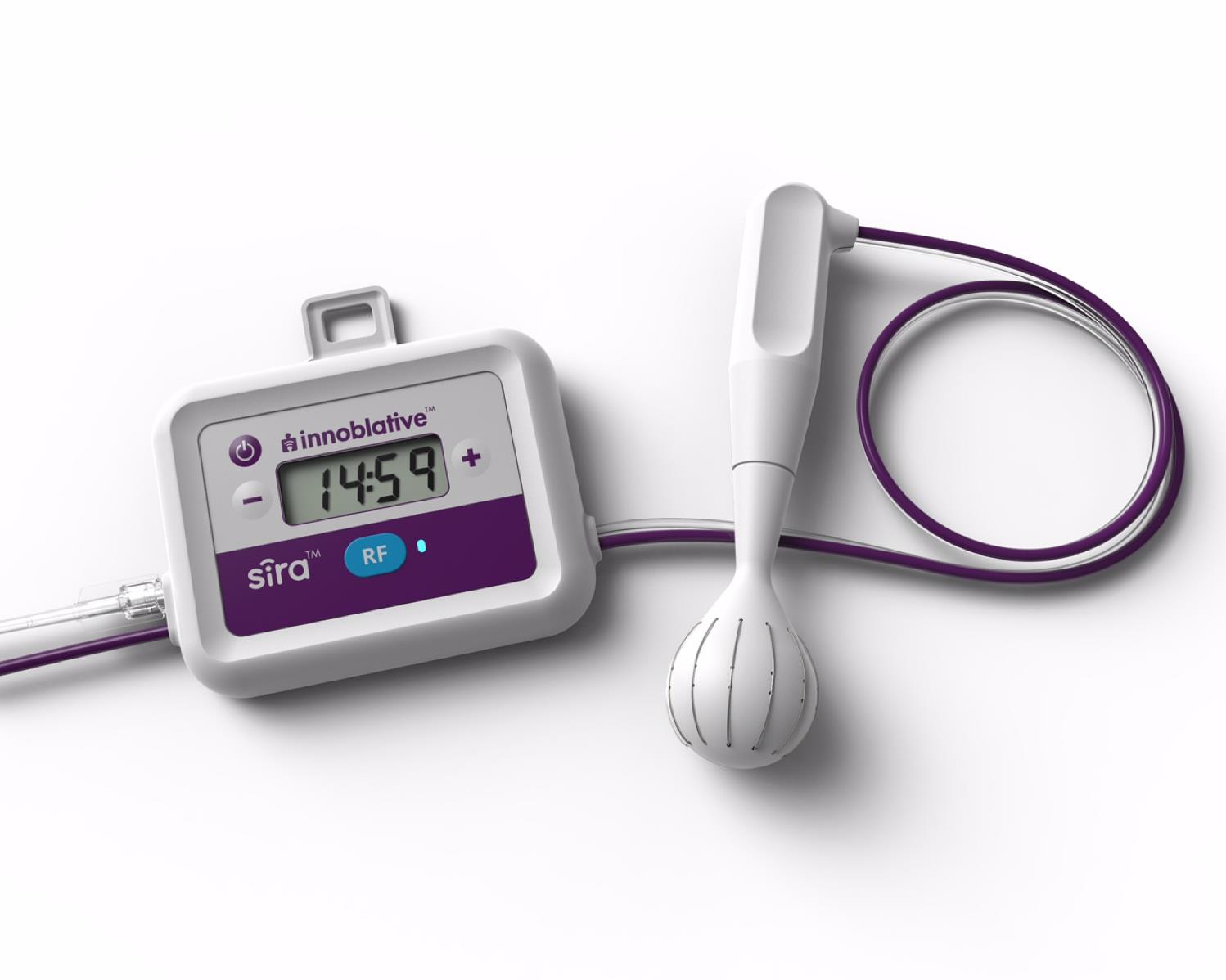
SIRA™ RFA Electrosurgical Device - Innoblative
The Problem
Innoblative’s SIRA device is a single-use, disposable applicator used in conjunction with a radiofrequency electrosurgical generator to effectively ablate target tissue. The device’s unique 4cm diameter spherical electrode creates a wide ablation zone, covering a larger surface area per ablation compared to devices currently available.
However, Innoblative’s early GLP preclinical studies raised serious concerns when the regulatory body made a request for additional information. The company attributed this disturbing development to insufficient attention to detail in their prior preclinical study.
“From that point, it was all hands on deck,” explained Tyler Wanke, Innoblative Co-Founder and CEO. “We needed the very best preclinical partners we could find to execute the study perfectly, among the highest compliance standards, protocols and extremely high levels of attention to detail.”
The Solution
It was clear that Innoblative needed to conduct their preclinical study perfectly to prove to the FDA that their technology was safe and effective, and they needed to engage the right partners to do this. A former-FDA regulatory consultant referred Innoblative to GCMI/T3 Labs to get the results they were looking for.
“The Atlanta medtech ecosystem is full of great hospitals and great surgeons,” said Tyler. “GCMI and T3 did a tremendous job with coordination, scheduling, flexibility and logistics to ensure those doctors felt comfortable and respected in a familiar environment and convenient location.
“This was a make-or-break moment for us. Having credentialed professionals managing the study, board certified veterinarians on staff and a team who cared about the project as if it was their own, is a distinguishing feature for GCMI/T3 Labs.”
The Results
Innoblative’s preclinical study with GCMI and T3 Labs resulted in a 510K submission that returned zero questions and a regulatory clearance within 1.5 months.
“We set a very aggressive set of timelines and endpoints,” Tyler continued. “The access to ecosystem resources, the staff qualifications, regimens on veterinary care and all-team coordination were paramount to achieving a GLP 510K submission that returned with zero questions asked in a very short timeframe.
“The GCMI/T3 Labs team helped us learn, were always well prepared and were intimately concerned with the project. It is more cost effective for companies and investors to get it right the first time. Go slightly slower when selecting the right set of studies, the right data sets, the right partner qualifications, and the odds on meeting your endpoint on or ahead of schedule increases.”
GEORGIA TECH BME FALL 2021 CAPSTONE: TEAM FIVE OF HEARTS
A Safer Way to Conduct Pericardiocentesis
SIRA™ RFA Electrosurgical Device - Innoblative
The Problem
Heart disease, Viral infections, autoimmune diseases, and cancer can all cause excess fluid to fill the pericardial sac. Physicians frequently perform “pericardiocentesis” by inserting a large 18-gauge needle through the patient’s chest and into the pericardial space to drain this fluid. Unfortunately, the needle can unintentionally perforate the vascular heart in up to 50% of these procedures, and 1 in 4 people die due to this complication.
The Solution
Team Five of Hearts devised the SafeCentesis, a device with a novel, half-coiled tip for parallel entry into the pericardium intending to allow physicians to more safely access and drain the pericardial space by entering the pericardium by no more than 1cm.
The Results
“In our tests supported by GCMI and T3 Labs, we found our device reduces pericardial entry depth by over three times less compared to currently available technologies including the 18 gauge needles on the market,” Lauren said. “This allows significantly more control over the depth of entry into the pericardial sac.”
EMORY CHILDREN’S PEDIATRIC RESEARCH CENTER / GEORGIA TECH BME
Re-expressing Pacemaking Cells for Cardiac Arrhythmia in Pediatric Patients

SIRA™ RFA Electrosurgical Device - Innoblative
The Problem
Children, especially newborns’ and infants’, heart rates are much faster than adults because their physiological needs are much higher. When a pacemaker is needed, the battery drains much faster. For congenital heart disease, patients require two to three high risk surgeries to replace the device to survive. Additionally, pacemaker wires can come off and break in small children who move around a great deal.
The Solution
By delivering a single gene, Dr. Cho’s technology can convert heart muscle into autonomously functioning pacemaker cells like the precious few ones we are born with that do not regenerate on their own. The research team have developed the technology to regenerate the pacemaking tissue with a single injection of a key gene.
The Results
“Our confidence in the data generated by our in vivo studies with T3 Labs cannot be greater,” Dr. Cho said. “The quality of the data leaves no uncertainty and a painstaking review of the 30–day continuous ECG data by two cardiologists has revealed no dangerous arrhythmias or safety concerns in the subject animals. The quality control and confidence in the data cannot be greater because those cardiologists reviewed the data bit by bit. It took seven months.”