Tearing down workforce development silos and supporting next-level collaboration for job growth
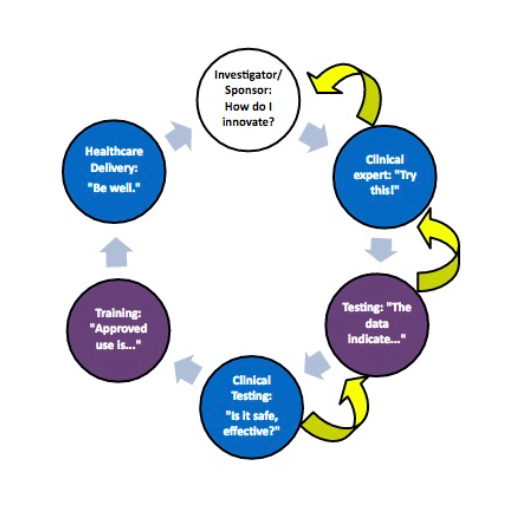
We have long maintained that the robust elements of Atlanta’s medtech ecosystem are ripe to support new, high-value job creation in Atlanta and throughout the southeastern United States. Through this lens of job creation through innovation, I was honored to be appointed for a second term on the National Advisory Council on Innovation and Entrepreneurship…