“Founded in 1917, the American Association for Thoracic Surgery (AATS), along with its philanthropic arm, the AATS Foundation, has sought to advance the field of cardiothoracic surgery. It is an international organization whose members have a proven record of distinction within the specialty and have made significant contributions to the care and treatment of cardiothoracic disease throughout the world.”
The 103rd! AATS Annual Meeting (AATS 2023) is May 6-9, 2023 at the Los Angeles County Convention Center. Check out the scientific program here.
 Founded by cardiologists for cardiologists, T3 Labs, a wholly owned subsidiary of the Global Center for Medical Innovation (GCMI) has been an industry leader in preclinical testing and bioskills training for novel cardiothoracic devices and technologies for more than two decades. We’re an AAALAC accredited facility that conducts feasibility, proof of concept, safety, and GLP medical device testing for studies in any therapeutic area and in small and large models. Since January 2012, we have archived more than 50 GLP studies for leading medical device manufacturers of which more than 25 products have received regulatory approvals.
Founded by cardiologists for cardiologists, T3 Labs, a wholly owned subsidiary of the Global Center for Medical Innovation (GCMI) has been an industry leader in preclinical testing and bioskills training for novel cardiothoracic devices and technologies for more than two decades. We’re an AAALAC accredited facility that conducts feasibility, proof of concept, safety, and GLP medical device testing for studies in any therapeutic area and in small and large models. Since January 2012, we have archived more than 50 GLP studies for leading medical device manufacturers of which more than 25 products have received regulatory approvals.
Our cardiology / cardiothoracic preclinical testing experience includes interventional, structural heart, electrophysiology, and renal nerve ablation technologies. Our preclinical, GLP work for leading cardiology/cardiothoracic device manufacturers employs a wide variety of techniques and models tailored to the test article.
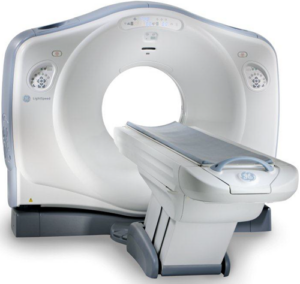 Recent news: GCMI recently acquired a GE LightSpeed VCT 64 Slice CT Scanner with cardiac gating capabilities. – https://gcmiatl.com/2023/02/21/onsite-state-of-the-art-ct-scanner-adds-capability-efficiency-time-and-cost-savings-for-gcmi-preclinical-customers/
Recent news: GCMI recently acquired a GE LightSpeed VCT 64 Slice CT Scanner with cardiac gating capabilities. – https://gcmiatl.com/2023/02/21/onsite-state-of-the-art-ct-scanner-adds-capability-efficiency-time-and-cost-savings-for-gcmi-preclinical-customers/
From the broader, 30,000 foot view, GCMI as a whole is the U.S. proving ground for global medical innovation. Our mission is to help direct the development, testing, training and commercialization of innovative medical products that improve quality based outcomes and delivery of healthcare for patients.
If you’re evaluating your new cardiothoracic technology’s readiness for preclinical work including pilot studies or if you just want to get together to talk about the latest in preclinical testing, bioskills training or medtech innovation across the board, email T3 Labs Director of Scientific Affairs Evan Goldberg to set up an onsite meeting at AATS 2023 in advance: evan.goldberg@t3labs.org
In our customers’ own words:
“Our experience with T3 Labs for the CardioMEMS device created new knowledge and refinements to the device that resulted in a product that was highly mature at the time we started our clinical trials in humans.” – Jason White, Senior Director of Product Development at St. Jude Medical

0063001-17SN
2017_06_30: Environmental Portrait of Hee Cheol Cho and his staff working at a lab in the Health Sciences Research Building on the Emory University Campus in Atlanta, GA. Stephen Nowland/Emory University
“Designing and managing the logistic demands of a large in vivo study is immensely complex and requires much more knowledge than a bench scientist’s,” he said. “That’s where the team at T3 Labs has been exceptional. They fill in all of the translational gaps for our requirements with very high levels of proficiency. The ability to execute cardiac cath lab preclinical programs combined with electrophysiology is exceedingly rare. T3 Labs has the infrastructure and expertise to make it happen.” – Dr. Hee Cheol Cho