Over 1,300 of the world’s foremost cardiothoracic surgeons comprise The American Association of Thoracic Surgery. It is the definition of an industry leading organization in thoracic surgery.
“Other than visiting a surgeon in his or her own operating room, there is no opportunity like [AATS] in education in thoracic surgery,” according to Charles D. Fraser, Jr., M.D.
Now in its 96th edition, T3 Labs is looking forward to attending the 2016 AATS Annual Meeting next week in Baltimore. We always gain new knowledge at AATS that advances our ability to deliver preclinical testing and training excellence for cardiovascular and cardiothoracic surgeons and those that support them.
Some of the top sessions we are looking forward to attending are The End of a Valve Surgery Era – Trans-Vascular Valve Rising, New Mechanical Circulatory Support Devices: An Update from Europe – HM III and MVAD on Sunday May 15, Innovations in Transcatheter Valve Therapies on Monday, May 16 and Cardiac Studies in Progress on Tuesday, May 17.
When it comes to cardiothoracic surgery, the implantable devices that support the surgeons and procedures and the training associated with those devices, T3 Labs is uniquely qualified to provide the highest levels of preclinical testing and training for cardiothoracic devices. T3 Labs is an industry leading preclinical CRO committed to excellence in translational research including preclinical cardiovascular research. After all, our program leaders are scientists, too.
We know GLP.
Our AAALAC accredited preclinical CRO has archived more than 50 GLP studies across all program areas from which more than 30 medical devices have achieved regulatory approval. GLP in 8 days? Yes, it’s possible.
We have the equipment and capabilities you need.
Proper navigation and placement of devices is critical. Our GE Vivid I Echo system enables perfect views of the heart for valve placements via intra-cardiac echocardiography (ICE), our fixed Philips Cathlab with a full CardioLab suite allows exceptional fluoroscopy and hemodynamic monitoring critical to placement. Access to CT and MRI allows the monitoring of devices such as valves and conduits. Additionally, we can support bypass procedures with our on-site perfusion systems, and technicians trained in running anesthesia for critical cardiothoracic cases. T3 Labs has the medical imaging, equipment and expertise needed to accelerate and maximize your investment in preclinical testing or training programs for cardiothoracic surgeons and their allied professional partners.
You need a trusted GLP partner.
For these reasons and more, like our staff of surgical specialists and DVMs, ALung Technologies relied on T3 Labs to help bring the the world’s first fully-integrated system for Respiratory Dialysis, a simple approach to extracorporeal CO2 removal (ECCO2R) closer to U.S. FDA approval.
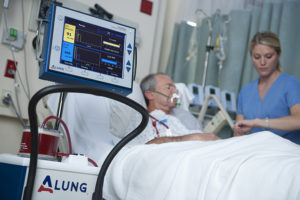 “T3’s recommendation for the model shift based on their experience and expertise was much more practical for this application based on our device’s capabilities and needs,” said Jeremy Kimmel, PhD Director of New Technology for ALung Technologies. “I am convinced this recommendation has accelerated our product refinement testing and preclinical trials saving ALung time and money at every step along the way.”
“T3’s recommendation for the model shift based on their experience and expertise was much more practical for this application based on our device’s capabilities and needs,” said Jeremy Kimmel, PhD Director of New Technology for ALung Technologies. “I am convinced this recommendation has accelerated our product refinement testing and preclinical trials saving ALung time and money at every step along the way.”
Are you evaluating your cardiothoracic device’s readiness for preclinical testing, need valuable feedback from clinicians, or the very best in surgical training for your device? Or do you just want to talk about the latest in cardiothoracic innovation? We want to connect with you at AATS 2016.
Contact T3 Labs Executive Director D. Andrew Stevenson (andrew.stevenson@emoryhealthcare.org) or Preclinical Testing Program Director Deepal Panchal (deepal.panchal@emoryhealthcare.org to arrange a meeting at AATS.
