Overheating lighting instruments coupled with human error are a leading cause of intraoperative fires and patient burns. The healthcare system refers to these as “never events” so they should never happen, right? But, data suggests that they occur more than twice a day. This was a red flag for co-founders James Rains and Kamil Makhnejia.
 At the Georgia Institute of Technology, Rains and Makhnejia began conceptualizing GloShield™ to address this safety issue. They then brought on to the team Dr. Spencer Kozinn, a local Atlanta urologist, who not only resonated with the safety issue being addressed, but also provided clinical expertise to the team.
At the Georgia Institute of Technology, Rains and Makhnejia began conceptualizing GloShield™ to address this safety issue. They then brought on to the team Dr. Spencer Kozinn, a local Atlanta urologist, who not only resonated with the safety issue being addressed, but also provided clinical expertise to the team.
Together, the future Jackson Medical team knew they had a great base concept but to successfully commercialize the idea, they would need an additional outside design and development perspective. That is when they engaged GCMI.
“While completing the Biomedical Innovation and Development Masters Program at Georgia Tech, I worked at GCMI, so when thinking about how to enhance GloShield and build upon the original concept for commercial success, I knew the Phase Zero program at GCMI would be the best option for us,” says Makhnejia.
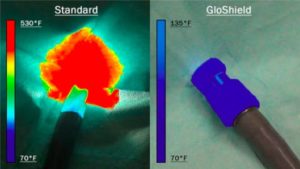
In 2017, the GCMI Phase Zero Program paved a pathway for Jackson Medical to further develop GloShield, an innovative light cable cap that reduces heat emissions in the operating room.
“During ‘Phase Zero,’ we ask and answer many questions to better understand a technology’s development pathway,” says Patrick Strane, Program Manager and Phase Zero lead for GCMI. “At the end of Phase Zero, an innovator will better understand what makes their technology function, what makes their concept unique, and what they need to do in a methodical, objective, stepwise manner to get that product through successive ‘phase gates’ into the hands of end-users.”
“GCMI helped to characterize our use need and the thermal environment, then prototyped solutions that met the more refined specifications,” says Rains. “We knew that we had an innovative concept and that there is nothing like GloShield currently on the market, but needed to ensure that we were taking the correct steps that would help us reach commercialization in a timely and cost-efficient manner.”
For Jackson Medical, GCMI’s Phase Zero program developed intellectual properties and a functioning prototype as well as received guidance on moving towards a commercial device.
“GloShield is us proving that we can bring a product to market and will set the stage for future innovations,” adds Rains.
Looking back, what advice would you give yourself in the early days of developing GloShield?
“In addition to GCMI, listen to the advice of the whole local medtech community,” says Rains. “In beginning this process, we were given valuable advice from seasoned entrepreneurs and innovators who have commercialized many medical products.
“Two mistakes we did make regarding timeline and funding – we found that things just take longer than they need to and cost more than you think they should. We estimated it would take ⅓ of the capital than it actually did. Luckily, because of organizations such as the Coulter Translational Foundation and the Georgia Research Alliance, we received the funds and also the mentorship we needed to propel us forward.
“And we are still engaged with GCMI to this day. We utilize GCMI for our office space. We work with the team constantly on GloShield’s development and by osmosis, we continue to learn. And, sure, the free coffee is nice, but the advice is more valuable.”
Jackson Medical now has three sales team members and are excited to officially launch GloShield nationwide in June 2018.
To learn more about Jackson Medical click here. To follow along with their successes via social media click here.
If you need help evaluating your medical product’s market landscape, regulatory pathway and whether or not it warrants further investment, email GCMI at info@devices.net.
