A new, helpful ‘tip’ for surgeons
A trocar is a medical device that is placed through the abdomen during laparoscopic surgery to act as a portal for the placement of other instruments during the procedure. This device usually has a metal or plastic sharpened or non-bladed tip, which can sometimes result in accidental perforation of internal organs upon entry.
Leading Lap has worked to change that by redesigning the trocar’s tip to prevent puncturing or injuring organs upon insertion. Georgia Tech biomedical engineering students Hailey Avis, Cameron Bennett, Will Kamnick and Katherine Strickland make up this team for the Fall 2019 Capstone Design Expo. The team hopes their new design will help mitigate risk for surgeons and reduce overall cost and time for these procedures.
“Our design is what we call ‘half-bladed,’” Strickland explained. “It has blades halfway up the side and a rubber tip because, ideally, rubber won’t pierce any internal organs.”
“Right now, a surgeon’s first entry with a trocar is kind of blind,” said Bennett. “If they accidentally stab one of the internal organs, then a simple surgery turns into a whole repair job.”
Bennett’s previous experience working in a cadaver lab revealed how frequently this issue occurred and played a key role in the team choosing this device for their project.
“Once we decided to focus on this issue, we began speaking to a lot of surgeons,” Bennett continued. “They told us that it’s something every surgeon is cautious about. Some of them had even seen extreme cases where something vital like the heart or liver was punctured.”
Verifying their solutions in a real OR
GCMI provided Leading Lap, along with five other biomedical engineering Capstone teams, full access to the OR at our preclinical facility to evaluate and test their device prototype in a cadaveric model. These facilities are normally used by medtech innovators for the testing of novel devices or by medical professionals for bioskills training.
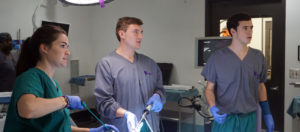
“I think this experience gave us a much better understanding of what we’re working with and what the clinical need is,” said Kamnick. “It’s one thing to see the numbers on paper, but it’s another thing to be in the OR doing it yourself. It gave us a better understanding of what our device needs to be able to withstand in order for it to function properly.”
Strickland added, “We’ve used tissue models before, but nothing compares to a real body. Just seeing it in action compared to what we’ve seen in the lab makes all the difference.”
