What you need, when you need it
For our cardiothoracic medical device testing and training sponsors, our GLP and AAALAC accredited preclinical CRO employs the most appropriate, efficient model and all equipment needed.
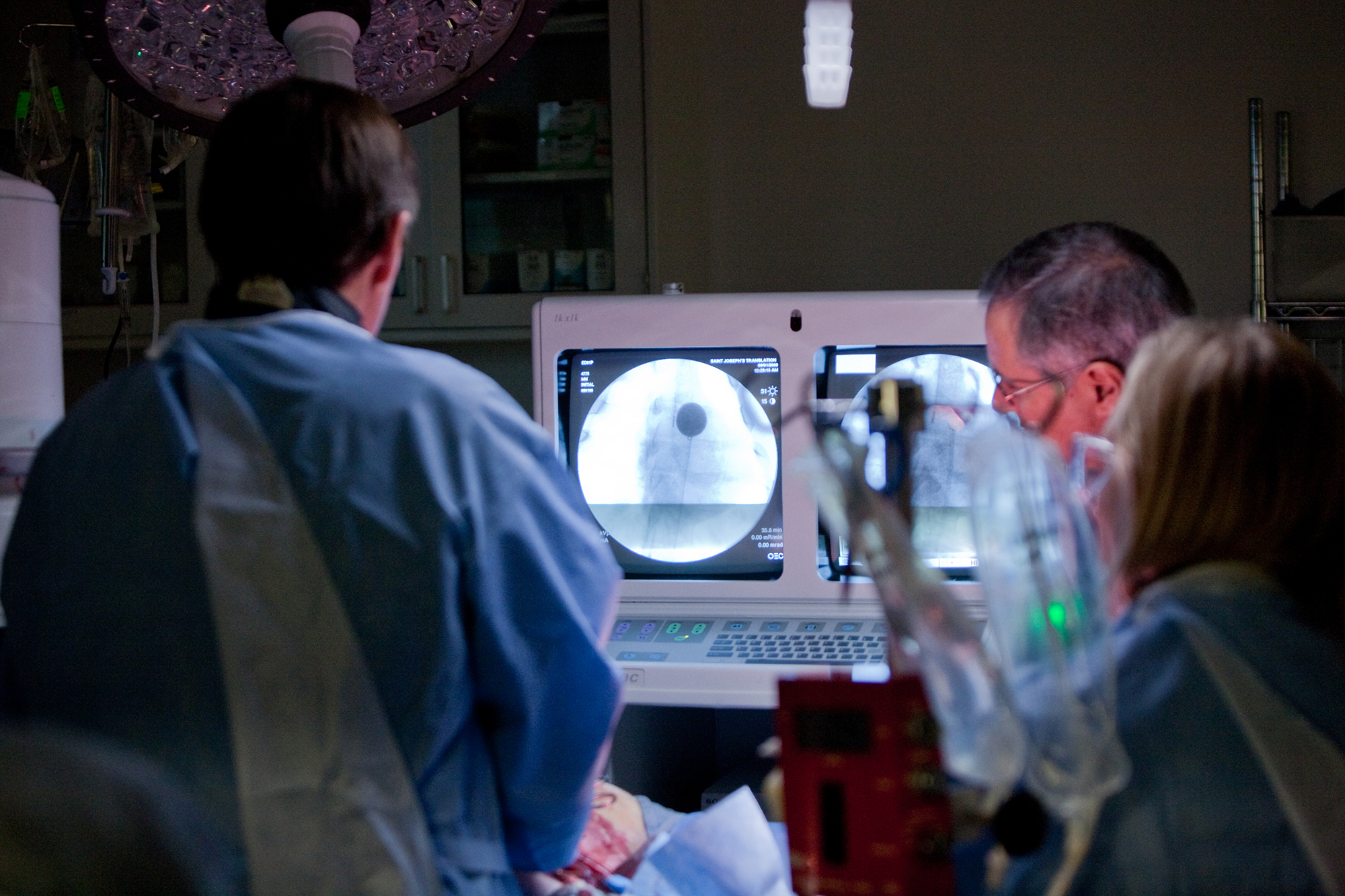
- C-arms
- ECHO including ICE, TTE and TEE
- Doppler
- IVUS
- OCT
- Micro-CT, MRI and CT
- Cardiothoracic Surgical Instrument trays
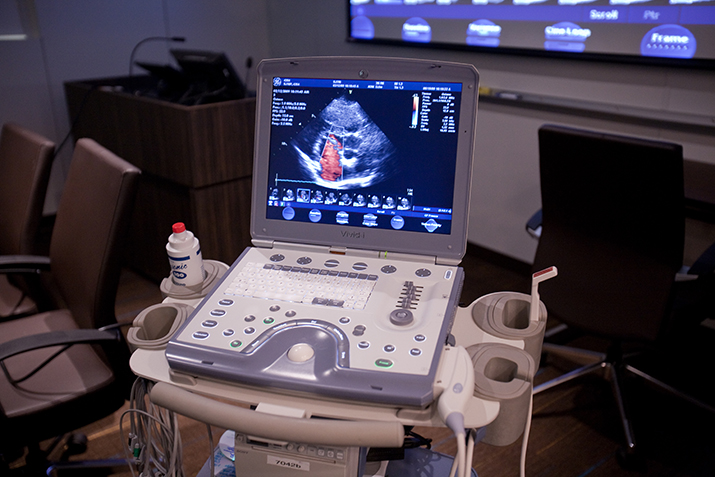
Our preclinical CRO program leaders are scientists as well
Our preclinical testing and training program leaders are trained scientists themselves, interested in understanding and participating in the science and methods behind therapeutic options in the cardiothoracic/cardiology space.
Recently, Program Director Irena Brants, along with Alizee pathology, published the first preclinical testing documentation, that a progressive regenerative response occurs as early as 7 days after renal denervation in “Circulation: Cardiovascular Interventions.”
Leading up to its regulatory approval and subsequent sale to St. Jude Medical, CardioMEMS relied on us for numerous proof-of-concept and feasibility studies for its groundbreaking wireless powered blood pressure implant capable of anticipating the onset of heart failure.
Experience
Our GLP preclinical program for cardiothoracic devices includes experience in models of ischemia, in vivo vascular function or reactivity and more in:
- MEMS,
- Interventional cardiology,
- Structural heart electrophysiology, and
- Renal nerve ablation technologies.
- Program managers have more than 50 years’ experience (all areas).
- 50 GLP studies archived
- 25 sponsor devices achieved regulatory approval (all types)

One seamless preclinical CRO engagement
Our relationships with Emory Healthcare, St. Joseph’s Medical, the Georgia Institute of Technology and GCMI (the Global Center for Medical Innovation) bring deep knowledge and create seamless simplicity and economy via one preclinical CRO engagement; eliminating the need for additional contracts with other physicians, CRO or labs.