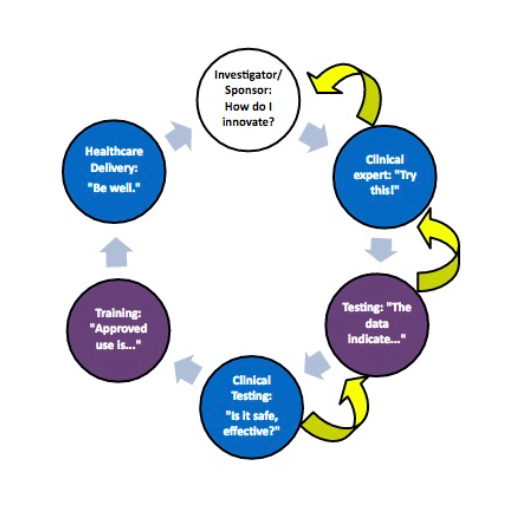
Medical device innovation does not come easy, or cheap. Nor should it. The stakes at every level are about as high as they come in innovation of any kind. From Concept to Cure, the seven stages of the medical device development collectively consume as many as seven years or more, tens of millions of dollars or more and no assurance of making it to market. Trust at every stage is critical.
The Seven Stages of Medical Device Development
- Research and Development
- Preclinical Testing
- Permission for Clinical Trial (510(k), IDE – Investigational Device Exemption, PMA – Premarket Notification Application)
- Clinical Trials
- Manufacturing
- Permission to Market
- Post Market Requirements
How do you know you’re ready and how do you make the most of your time and investment during preclinical medical device testing?
Here are the top 5 things medical device manufacturers need to know in order to achieve successful preclinical testing, secure FDA clearance and advance to the clinic.
Identifying a trusted preclinical lab
Are your prospective CRO candidates GLP compliant and AAALAC accredited? The FDA usually recommends that preclinical studies performed in support of submissions, 510(k) or IDE, are in compliance with 21 CFR Part 58. Does the lab have the expertise to perform your study? For example: study directors with advanced degrees and direct knowledge of your technology. T3 Labs study directors come from biomedical engineering and veterinary backgrounds enabling them to more intimately understand a new medical device’s intentions, prospective value and the most efficient path forward in the FDA regulatory process.
Program and protocol development: employing the right model
Are you using the most cost-efficient model? Have you had any pre-submission discussions with the FDA and possess a study design in mind? We frequently field requests from sponsors for certain, specific in vivo models when there may be other alternatives that are not only more efficient, but also generate a cleaner data set. We highly recommend performing pilot studies before GLP studies so that your study is clean and submission ready.
Study execution: the need for transparency and timelines
Has your preclinical CRO made the timeline as clear as possible? Can they validate the timeline with successful examples? We have seen preclinical studies completed from beginning to end in as few as 2-8 weeks, or as many as 16-52 weeks or more. Further, our hiring procedures, individualized personnel development, Team-based Targeted Training programs, and our customer-focused service model ensures that we will deliver on-time, on- cost and on-quality. And you will know the status every step of the way via our secure and Sponsor-specific Box.com accounts where your data is at your finger tips.
Reports: what do the best include?
Reports for GLP and non-GLP studies should enable third parties to reconstruct studies (if necessary) many years after completion. The best reports include study objectives, study design, test and control articles used, methods, veterinary care, raw results and a robust, factual conclusion.
QAU & Audits
GLP compliant preclinical laboratories must have a quality assurance unit (QAU). The QAU must be independent of test facility management and should be able to review and audit various aspects of the study. The QAU also ensures that standard operating procedures (SOP) are in place for everything from veterinary care to equipment use. Before placing a GLP study, medical device manufacturers are encouraged to audit the prospective preclinical lab.
No Questions Asked, T3 Labs knows Stage II: Preclinical Medical Device Testing
Since 2012, T3 Labs has archived more than 50 GLP studies for leading medical device manufacturers of which more than 30 products have cleared the FDA medical device review process and received FDA approval. Our colleagues at Surefire Medical recently enjoyed this experience for their direct-to-tumor treatment delivery device Surefire Precision. The vast majority of T3 preclinical GLP studies return with zero questions asked.
A comprehensive view of medical device innovation
T3 Labs’ expertise does not end at Preclinical Testing. We have a comprehensive understanding of Stage I – Research and Development, via our affiliation with GCMI (Global Center for Medical Innovation), the Southeast’s first and only comprehensive medical device innovation center.
Once your product is on the market, we can help you come full circle with our physician and sales force training programs. T3 Labs provides medical device testing and training for more than 2,000 physicians, medical device sales force members, students and other allied healthcare professionals every year.
Talk to us about your preclinical medical device testing and training needs
If your company is evaluating a product’s readiness for FDA approval, including preclinical medical device testing, contact our team at 404-251-0600 or T3Labs@Emory.edu Connect with T3 Labs on LinkedIn.