Medical technology innovators learn quickly that there is little, if any, flexibility in the FDA approval process. NFANT Labs knew they needed multidisciplinary partners able to build flexibility into an inflexible process, designing and developing their product with a constant eye on efficiency and the FDA approval prize.
In the United States, up to 70% of infants born prematurely and 10% of infants born full term have trouble transitioning to breast or bottle feeding on their own; infant feeding complications are one of the leading causes of delay in discharge from the NICU. Furthermore, infants that don’t reach feeding independence are at risk for returning to the NICU.
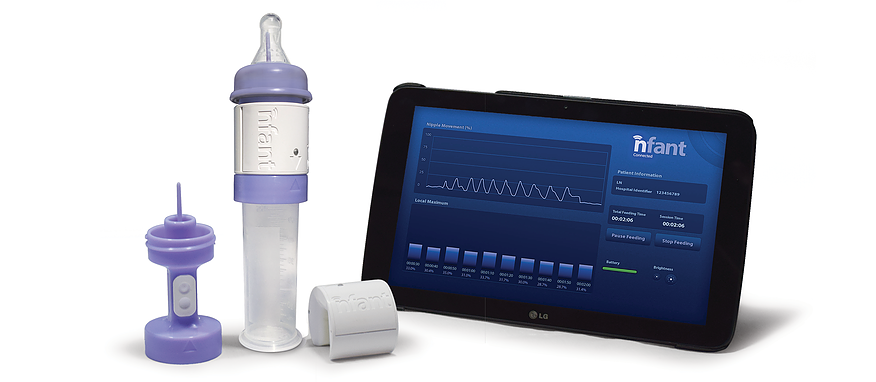
NFANT Labs has created a device that tracks infant progress in feeding. nfant® Feeding Solution is a system that provides data to help clinicians guide premature infants to a safe and healthy transition to independent feeding. The sensor, which connects to standard nipples and bottles, feeds data instantly to the clinician’s tablet, allowing them to see the impact of their therapies and tailor care for each infant.
Lou Malice, CEO of NFANT Labs, is a seasoned medtech professional who knows that innovation requires a great team. NFANT engaged GCMI early in the developmental pathway.
It started in a crowded 10’ x 10’ cubicle. Malice explains, ”The decision to move into GCMI’s space provided instant access to the right people and right services for the right price at the right time.” He and Tommy Cunningham, Co-founder and COO of NFANT, knew that GCMI could help the company achieve a minimum viable product.
A “just in time” approach to medical device development
GCMI provided the design for the plastic parts that make up the nfant®Feeding Solution’s innovative device and also introduced the NFANT team to electrical engineering and software experts that helped develop the app. In each step of the way, GCMI aided the prototyping and 3D printing of the device.
“GCMI was incredibly flexible with us,” Malice says. “We could essentially be a la carte with their services, such as design, machine shop, software and documentation. This was ideal given that, like many medical device startups, we had a limited budget and only needed certain services.”
GCMI combines internal capabilities in conceptualization, design, engineering and prototyping with external medtech resources such as IP experts and regulatory consultants. The goal is to save startups and innovators time and money by anticipating their needs and connecting them to value added partners at the right time.
“If we worked with another design and development firm, we wouldn’t have had the flexibility to do what we needed to do in the time frame we set,” Malice says. “GCMI is unique in that we were able to choose the services that we needed precisely at the time we needed them. The way GCMI engages with their customers is unlike any other firm.
“We would often say to the GCMI team ‘we need help creating this prototype, and, by the way, we need it by Friday.’ The GCMI team always delivered and their sensitivity to time and budget allowed us to take our device from ideation to FDA submission in 10 months.”
Advice from Lou Malice on how to approach the commercialization process
- Have the funding and understand your market. Innovators frequently want to start prototyping right away without answering critical questions like, Who is going to fund the project? What does the market for this device look like? How are you going to get reimbursed for it?
- Understand that medtech innovation may start with an idea, but it is a rigorous process. Many medtech startups think that the ideation is the difficult part and that is simply not the case. It only gets more difficult from there. Find flexible partners who can help you navigate the process.
- There are many steps one must take in order to commercialize a medical product and you cannot skip steps because you will eventually end up back at square one – you don’t get to decide which steps you want to do.
“GCMI stresses these points to every innovator,” said Malice. “There is no flexibility in the FDA process. GCMI makes sure you check all of the boxes because at the end of the day, in order to innovate and improve patient outcomes for the betterment of the entire healthcare community, you have to first make the FDA happy.”
What’s next for NFANT Labs?
nfant® is currently used in 12 hospitals with another 51 healthcare providers looking to adopt the device in their NICU. NFANT’s data repository grows each time the device is used and they look to leverage this proprietary data in the future to devise treatment plans and NICU benchmarking using predictive analytics. NFANT is seeking investors for their series B round of $5M to take their digital health concept to the next level.
GCMI and T3 Labs is an all-in-one resource for medical device innovators: bringing together the experts of the commercialization process, GCMI, and the professionals at T3 who are able to guide innovators through preclinical testing in an AAALAC accredited and GLP compliant facility.
If you are a physician innovator or engineer currently developing a novel medical technology, or if you have an idea and want to know how to navigate from concept to cure to commercialization, we want to be your trusted partner! Contact GCMI via email or give us a call at 404-385-5191.