Announced recently via the Georgia Tech News Center, Microneedle Patches for flu vaccination were found safe to use following a successful human clinical trial.
“Despite the potentially severe consequences of illness and even death, only about 40 percent of adults in the United States receive flu shots each year,” writes Holly Korschun, Director, Research Communications at Emory University. “However, researchers believe a new self-administered, painless vaccine skin patch containing microscopic needles could significantly increase the number of people who get vaccinated.”
Announced recently via the Georgia Tech News Center, the microneedle patch for flu vaccination, a collaboration between Emory, Georgia Tech, and GCMI, was found safe to use following a successful human clinical trial. The research was supported by the National Institute of Biomedical Imaging and Bioengineering of the National Institutes of Health.
“A phase I clinical trial conducted by Emory University in collaboration with researchers from the Georgia Institute of Technology and GCMI has found that influenza vaccination using Band-Aid-like patches with dissolvable microneedles was safe and well-tolerated by study participants, was just as effective in generating immunity against influenza, and was strongly preferred by study participants over vaccination with a hypodermic needle and syringe.
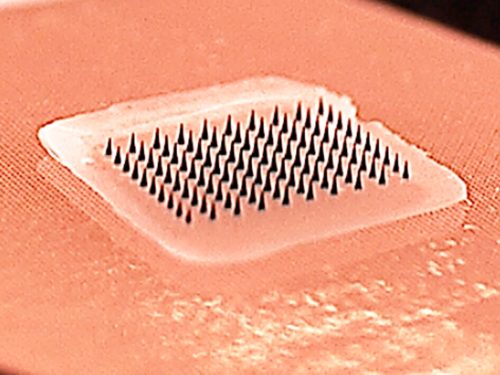
The microneedle patch vaccine could also save money because it is easily self-administered, could be transported and stored without refrigeration, and is easily disposed of after use without sharps waste.”
“Watching the Micron team work so hard to achieve their goals is a benchmark for other start up companies to model after,” says Patrick Strane, Sr. Project Engineer at GCMI. “It’s also an added bonus that they are a pleasure to have around the office!”
“The researchers are also working to develop microneedle patches for use with other vaccines, including measles, rubella and polio.”
We want to extend a huge congratulations to the entire team responsible for developing the microneedle patch- we are excited for the work that is still to come.
You can read the article, in full, here.
The Global Center of Medical Innovation (GCMI) and its wholly owned subsidiary T3 Labs, is the world’s leader in driving efficient medical product innovation. Using our proven high-quality processes, our expert staff works alongside physician innovators, hospital teams, Fortune 500s, start-ups, academic and government funded innovators every day to commercialize innovative medical devices and products, like the microneedle patch, that improve quality based outcomes and delivery of healthcare for patients.
If you are a physician innovator with an idea for an innovative medical device, a company gauging your product’s readiness for preclinical, or seeking a partner in gaining FDA approval, contact GCMI and T3 Labs today to learn how our expert team can help you take your concept through cure, preclinical testing, and commercialization as well as provide surgeons the bioskills training necessary for your innovation.
